The Intestinal Epithelium – Fluid Fate and Rigid Structure from Crypt Bottom to Villus Tip
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study of the Ultrastructure of Microvilli in the Epithelium of Small and Large Intestine of Mice
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central A COMPARATIVE STUDY OF THE ULTRASTRUCTURE OF MICROVILLI IN THE EPITHELIUM OF SMALL AND LARGE INTESTINE OF MICE T. M. MUKHERJEE and A. WYNN WILLIAMS From the Electron Microscope Laboratory, the Departlnent of Pathology, the University of Otago Medical School, Dunedin, New Zealand ABSTRACT A comparative analysis of the fine structure of the microvilli on jejunal and colonic epi- thelial cells of the mouse intestine has been made. The microvilli in these two locations demonstrate a remarkably similar fine structure with respect to the thickness of the plasma membrane, the extent of the filament-free zone, and the characteristics of the microfila- ments situated within the microvillous core. Some of the core microfilaments appear to continue across the plasma membrane limiting the tip of the microvillus. The main differ- ence between the microvilli of small intestine and colon is in the extent and organization of the surface coat. In the small intestine, in addition to the commonly observed thin surface "fuzz," occasional areas of the jejunal villus show a more conspicuous surface coat covering the tips of the microvilli. Evidence has been put forward which indicates that the surface coat is an integral part of the epithelial cells. In contrast to the jejunal epithelium, the colonic epithelium is endowed with a thicker surface coat. Variations in the organization of the surface coat at different levels of the colonic crypts have also been noted. The func- tional significance of these variations in the surface coat is discussed. -

Gastrointestinal Stem Cells in Health and Disease: from Flies to Humans Hongjie Li1,2 and Heinrich Jasper1,*
© 2016. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2016) 9, 487-499 doi:10.1242/dmm.024232 REVIEW SUBJECT COLLECTION: TRANSLATIONAL IMPACT OF DROSOPHILA Gastrointestinal stem cells in health and disease: from flies to humans Hongjie Li1,2 and Heinrich Jasper1,* ABSTRACT is Barrett’s metaplasia (see Box 1), in which the esophageal The gastrointestinal tract of complex metazoans is highly squamous epithelium acquires properties that are reminiscent of the compartmentalized. It is lined by a series of specialized epithelia gastric or intestinal columnar epithelium. This transformation has that are regenerated by specific populations of stem cells. To maintain been associated with acid reflux disease and is believed to be a cause tissue homeostasis, the proliferative activity of stem and/or progenitor of esophageal adenocarcinomas (Falk, 2002; Hvid-Jensen et al., cells has to be carefully controlled and coordinated with regionally 2011). Dysplasia (see Box 1), another type of epithelial lesion that distinct programs of differentiation. Metaplasias and dysplasias, commonly affects the human GI tract, is characterized by aberrant precancerous lesions that commonly occur in the human cell proliferation and differentiation. Dysplastic changes are often gastrointestinal tract, are often associated with the aberrant found at later stages during epithelial carcinogenesis than are proliferation and differentiation of stem and/or progenitor cells. The metaplasias, and eventually lead to invasive carcinoma (see Box 1) increasingly sophisticated characterization of stem cells in the (Correa and Houghton, 2007; Ullman et al., 2009). Much remains to gastrointestinal tract of mammals and of the fruit fly Drosophila has be learnt about intestinal metaplasias and dysplasias, not least provided important new insights into these processes and into the because of their clinical significance, such as the exact cellular mechanisms that drive epithelial dysfunction. -

Study of Mucin Turnover in the Small Intestine by in Vivo Labeling Hannah Schneider, Thaher Pelaseyed, Frida Svensson & Malin E
www.nature.com/scientificreports OPEN Study of mucin turnover in the small intestine by in vivo labeling Hannah Schneider, Thaher Pelaseyed, Frida Svensson & Malin E. V. Johansson Mucins are highly glycosylated proteins which protect the epithelium. In the small intestine, the goblet Received: 23 January 2018 cell-secreted Muc2 mucin constitutes the main component of the loose mucus layer that traps luminal Accepted: 26 March 2018 material. The transmembrane mucin Muc17 forms part of the carbohydrate-rich glycocalyx covering Published: xx xx xxxx intestinal epithelial cells. Our study aimed at investigating the turnover of these mucins in the small intestine by using in vivo labeling of O-glycans with N-azidoacetylgalactosamine. Mice were injected intraperitoneally and sacrifced every hour up to 12 hours and at 24 hours. Samples were fxed with preservation of the mucus layer and stained for Muc2 and Muc17. Turnover of Muc2 was slower in goblet cells of the crypts compared to goblet cells along the villi. Muc17 showed stable expression over time at the plasma membrane on villi tips, in crypts and at crypt openings. In conclusion, we have identifed diferent subtypes of goblet cells based on their rate of mucin biosynthesis and secretion. In order to protect the intestinal epithelium from chemical and bacterial hazards, fast and frequent renewal of the secreted mucus layer in the villi area is combined with massive secretion of stored Muc2 from goblet cells in the upper crypt. Te small intestinal epithelium is constantly aiming to balance efective nutritional uptake with minimal damage due to exposure to ingested, secreted and resident agents. -

Transcytosis of Listeria Monocytogenes Across The
Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, Marc Lecuit To cite this version: Georgios Nikitas, Chantal Deschamps, Olivier Disson, Théodora Niault, Pascale Cossart, et al.. Tran- scytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. Journal of Experimental Medicine, Rockefeller University Press, 2011, 208 (11), pp.2263-2277. 10.1084/jem.20110560. pasteur-02040395 HAL Id: pasteur-02040395 https://hal-pasteur.archives-ouvertes.fr/pasteur-02040395 Submitted on 20 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Published Online: 3 October, 2011 | Supp Info: http://doi.org/10.1084/jem.20110560 Downloaded from jem.rupress.org on February 19, 2019 Article Transcytosis of Listeria monocytogenes across -

Nanoarchitecture and Dynamics of the Mouse Enteric Glycocalyx Examined by Freeze-Etching Electron Tomography and Intravital Microscopy
ARTICLE https://doi.org/10.1038/s42003-019-0735-5 OPEN Nanoarchitecture and dynamics of the mouse enteric glycocalyx examined by freeze-etching electron tomography and intravital microscopy Willy W. Sun1,2,5, Evan S. Krystofiak1,5, Alejandra Leo-Macias1, Runjia Cui1, Antonio Sesso3, Roberto Weigert 4, 1234567890():,; Seham Ebrahim4 & Bechara Kachar 1* The glycocalyx is a highly hydrated, glycoprotein-rich coat shrouding many eukaryotic and prokaryotic cells. The intestinal epithelial glycocalyx, comprising glycosylated transmembrane mucins, is part of the primary host-microbe interface and is essential for nutrient absorption. Its disruption has been implicated in numerous gastrointestinal diseases. Yet, due to chal- lenges in preserving and visualizing its native organization, glycocalyx structure-function relationships remain unclear. Here, we characterize the nanoarchitecture of the murine enteric glycocalyx using freeze-etching and electron tomography. Micrometer-long mucin filaments emerge from microvillar-tips and, through zigzagged lateral interactions form a three-dimensional columnar network with a 30 nm mesh. Filament-termini converge into globular structures ~30 nm apart that are liquid-crystalline packed within a single plane. Finally, we assess glycocalyx deformability and porosity using intravital microscopy. We argue that the columnar network architecture and the liquid-crystalline packing of the fila- ment termini allow the glycocalyx to function as a deformable size-exclusion filter of luminal contents. 1 Laboratory of Cell Structure and Dynamics, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, MD 20892, USA. 2 Neuroscience and Cognitive Science Program, University of Maryland, College Park, MD 20740, USA. 3 Sector of Structural Biology, Institute of Tropical Medicine, University of São Paulo, Sao Paulo, SP 05403, Brazil. -

Regulation of Intestinal Blood Flow
Journal of Surgical Research 93, 182–196 (2000) doi:10.1006/jsre.2000.5862, available online at http://www.idealibrary.com on RESEARCH REVIEW Regulation of Intestinal Blood Flow Paul J. Matheson, Ph.D.,*,†,1 Mark A. Wilson, M.D., Ph.D.,*,†,‡ and R. Neal Garrison, M.D.*,†,‡ *Center for Excellence in Applied Microcirculatory Research and ‡Department of Surgery, University of Louisville, Louisville, Kentucky 40292; and †Louisville Veterans Affairs Medical Center, Louisville, Kentucky 40206 Submitted for publication July 29, 1999 arteries is typically 20–25% of cardiac output in the The gastrointestinal system anatomically is posi- unfed state [2]. There is extensive overlap or collateral tioned to perform two distinct functions: to digest and circulation in the distal vascular distributions of these absorb ingested nutrients and to sustain barrier func- arteries. During nutrient absorption, blood flow in each tion to prevent transepithelial migration of bacteria of these arteries is increased sequentially as the diges- and antigens. Alterations in these basic functions con- tive chyme passes over the mucosal surface supplied by tribute to a variety of clinical scenarios. These pri- mary functions intrinsically require splanchnic blood the particular arteries [3]. Following nutrient absorp- flow at both the macrovascular and microvascular lev- tion, the blood flow to each segment returns to baseline els of perfusion. Therefore, a greater understanding of levels as the chyme moves past that region of the the mechanisms that regulate intestinal vascular per- digestive tract [4, 5]. This postprandial increase in fusion in the normal state and during pathophysiolog- blood flow is independent of organ distention and is ical conditions would be beneficial. -
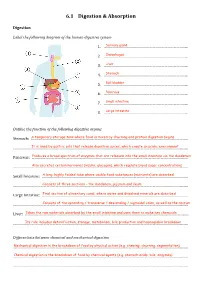
Topic 6.1 Answers
6.1 Digestion & Absorption Digestion Label the following diagram of the human digestive system 1. ………………………………………………………………………...Salivary gland 2. ………………………………………………………………………...Oesophagus 3. ………………………………………………………………………...Liver 4. ………………………………………………………………………...Stomach 5. ………………………………………………………………………...Gall bladder 6. ……Pancreas…………………………………………………………………... 7. ………………………………………………………………………...Small intestine 8. ………………………………………………………………………...Large intestine Outline the function of the following digestive organs Stomach: ………………………………………………………………………………………A temporary storage tank where food is mixed by churning and………………………………………………… protein digestion begins …………………………………………………………………………………………………………………………………………………..........It is lined by gastric pits that release digestive juices, which create an acidic environment Pancreas: …………..……………………………………………………………………………………………………………………………Produces a broad spectrum of enzymes that are released into the small intestine via the duodenum …………………………………………………………………………………………………………………………………………………..........Also secretes certain hormones (insulin, glucagon), which regulate blood sugar concentrations Small Intestine: …………….……………………………………………………………………………………………A long, highly folded tube where usable food substances (nutrients) are absorbed…………………… …………………………………………………………………………………………………………………………………………………..........Consists of three sections – the duodenum, jejunum and ileum Large Intestine: …………….…………………………………………………………………………………………………………………Final section of alimentary canal, where water and dissolved minerals are absorbed …………………………………………………………………………………………………………………………………………………..........Consists -

The Intestinal Stem Cell
Downloaded from genesdev.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW The intestinal stem cell Nick Barker, Marc van de Wetering, and Hans Clevers1 Hubrecht Institute and University Medical Center Utrecht, Uppsalalaan 8, 3584CT Utrecht, the Netherlands The epithelium of the adult mammalian intestine is in a Claudinot et al. 2005). Of note, multipotency can only be constant dialog with its underlying mesenchyme to di- definitively demonstrated when transplantation can be rect progenitor proliferation, lineage commitment, ter- performed with a single cell, which is rarely possible. As minal differentiation, and, ultimately, cell death. The an alternative strategy, candidate stem cells are geneti- epithelium is shaped into spatially distinct compart- cally marked in situ, after which the introduced marker ments that are dedicated to each of these events. While allows the visualization of the modified stem cell and its the intestinal epithelium represents the most vigorously clonal offspring over time. As an example of the latter renewing adult tissue in mammals, the stem cells that approach, a progesterone-responsive version of the Cre fuel this self-renewal process have been identified only recombinase enzyme was specifically expressed in cells recently. The unique epithelial anatomy makes the in- residing in the bulge region of hair follicles using a trans- testinal crypt one of the most accessible models for the genic Keratin-15 promoter (Morris et al. 2004). Activa- study of adult stem cell biology. This review attempts to tion of the Cre enzyme by progesterone irreversibly ac- provide a comprehensive overview of four decades of re- tivated the genetic marker R26R-LacZ in the bulge cells. -

Intestinal Organoids Generated from Human Pluripotent Stem Cells
DOI: 10.31662/jmaj.2019-0027 https://www.jmaj.jp/ Review Article Intestinal Organoids Generated from Human Pluripotent Stem Cells Satoru Tsuruta1),2), Hajime Uchida3), and Hidenori Akutsu2) Abstract: The gastrointestinal system is one of the most complex organ systems in the human body, and consists of numerous cell types originating from three germ layers. To understand intestinal development and homeostasis and elucidate the patho- genesis of intestinal disorders, including unidentified diseases, several in vitro models have been developed. Human pluripo- tent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have remarkable developmental plasticity and possess the potential for a wide variety of applications. Three-dimensional organs, termed organoids and produced in vitro by PSCs, contain not only epithelium but also mesenchymal tissue and partially recapitu- late intestinal functions. Such intestinal organoids have begun to be applied in disease models and drug development and have contributed to a detailed analysis of molecular interactions and findings in the synergistic development of biomedicine for human digestive organs. In this review, we describe gastrointestinal organoid technology derived from PSCs and consid- er its potential applications. Key Words: intestinal organoids, embryonic stem cells, induced pluripotent stem cells, gastrointestinal disease, drug discovery Introduction the anterior to posterior axis. Accompanied by the develop- ment of the fetus, repeated gut -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -

The Number of Villi in Rat's Jejunum and Ileum: Effect of Normal Growth, Partial Enterectomy, and Tube Feeding
J. Anat. (1972). 111, 2, pp. 283-291 283 With 6 figures Printed in Great Britain The number of villi in rat's jejunum and ileum: effect of normal growth, partial enterectomy, and tube feeding J. M. FORRESTER Department of Physiology, Edinburgh University (Accepted 8 January 1972) INTRODUCTION The villi of the rat small intestine are covered by enterocytes which have a life-span, from their time of origin in the crypts until shedding at the villus tip, of only about one and a half days (Leblond & Stevens, 1948; Bertalanffy, 1960). Their shape varies from one part of the small intestine to another, and even adjacent villi may differ strikingly. In view of these features suggesting a rapidly changing scene, this paper describes a procedure for enumerating the villi in rat jejunum and ileum, and ex- amines the stability of the total number during normal growth, after partial enter- ectomy, and after tube feeding. METHODS Locally bred Wistar male rats were used. They were fed on a standard pelleted rat food manufactured in Edinburgh. They always had tap water ad libitum. Enumeration procedure. Rats were killed by inhalation of chloroform in the morn- ing. The position of the suspensory ligament was marked on the small intestine where a band of connective tissue is attached to the intestine at the duodenojejunal junction. Then the small intestine was removed from pylorus to ileocolic valve by gentle traction, and washed through with cold saline (NaCl 0-9 %, w/v). It was weighed, and after removal of the duodenum, was laid in a trough and perfused with Bouin's solution at an outlet pressure of 20 cm of solution for at least 20 minutes (Hromadkova & Skala, 1969). -

Regulation of Colonic Epithelial Cell Homeostasis by Mtorc1
www.nature.com/scientificreports OPEN Regulation of colonic epithelial cell homeostasis by mTORC1 Takenori Kotani1, Jajar Setiawan1,2, Tasuku Konno1, Noriko Ihara1, Saki Okamoto1, Yasuyuki Saito1, Yoji Murata1, Tetsuo Noda3 & Takashi Matozaki1* Cell signaling important for homeostatic regulation of colonic epithelial cells (CECs) remains poorly understood. Mammalian target of rapamycin complex 1 (mTORC1), a protein complex that contains the serine-threonine kinase mTOR, mediates signaling that underlies the control of cellular functions such as proliferation and autophagy by various external stimuli. We here show that ablation of tuberous sclerosis complex 2 (Tsc2), a negative regulator of mTORC1, specifcally in intestinal epithelial cells of mice resulted in increased activity of mTORC1 of, as well as increased proliferative activity of, CECs. Such Tsc2 ablation also reduced the population of Lgr5-positive colonic stem cells and the expression of Wnt target genes in CECs. The stimulatory phosphorylation of the kinase Akt and inhibitory phosphorylation of glycogen synthase kinase 3β were both markedly decreased in the colon of the Tsc2 conditional knockout (CKO) mice. Development of colonic organoids with cryptlike structures was enhanced for Tsc2 CKO mice compared with control mice. Finally, Tsc2 CKO mice manifested increased susceptibility to dextran sulfate sodium–induced colitis. Our results thus suggest that mTORC1 activity promotes the proliferation of, as well as the expression of Wnt target genes in, CECs and thereby contributes to colonic organogenesis and homeostasis. Both the small intestine and colon of mammals are important for food digestion and the absorption of nutrients, water, and electrolytes. Te intestinal epithelium, in particular, plays a key role in these functions, although the structure and cell components of the epithelium difer between the small intestine and colon.