Germ Line Versus Soma in the Transition from Egg to Embryo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
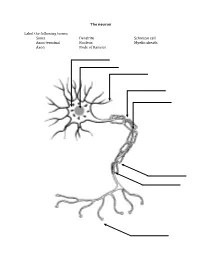
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Sex Determination in Mammalian Germ Cells: Extrinsic Versus Intrinsic Factors
REPRODUCTIONREVIEW Sex determination in mammalian germ cells: extrinsic versus intrinsic factors Josephine Bowles and Peter Koopman Division of Molecular Genetics and Development, and ARC Centre of Excellence in Biotechnology and Development, Institute for Molecular Bioscience, The University of Queensland, Brisbane, Queensland 4072, Australia Correspondence should be addressed to J Bowles; Email: [email protected] Abstract Mammalian germ cells do not determine their sexual fate based on their XX or XY chromosomal constitution. Instead, sexual fate is dependent on the gonadal environment in which they develop. In a fetal testis, germ cells commit to the spermatogenic programme of development during fetal life, although they do not enter meiosis until puberty. In a fetal ovary, germ cells commit to oogenesis by entering prophase of meiosis I. Although it was believed previously that germ cells are pre-programmed to enter meiosis unless they are actively prevented from doing so, recent results indicate that meiosis is triggered by a signaling molecule, retinoic acid (RA). Meiosis is avoided in the fetal testis because a male-specifically expressed enzyme actively degrades RA during the critical time period. Additional extrinsic factors are likely to influence sexual fate of the germ cells, and in particular, we postulate that an additional male-specific fate-determining factor or factors is involved. The full complement of intrinsic factors that underlie the competence of gonadal germ cells to respond to RA and other extrinsic factors is yet to be defined. Reproduction (2010) 139 943–958 Introduction A commitment to oogenesis involves pre-meiotic DNA replication and entry into and progression through Germ cells are the special cells of the embryo that prophase of the first meiotic division during fetal life. -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Gene Therapy Glossary of Terms
GENE THERAPY GLOSSARY OF TERMS A • Phase 3: A phase of research to describe clinical trials • Allele: one of two or more alternative forms of a gene that that gather more information about a drug’s safety and arise by mutation and are found at the same place on a effectiveness by studying different populations and chromosome. different dosages and by using the drug in combination • Adeno-Associated Virus: A single stranded DNA virus that has with other drugs. These studies typically involve more not been found to cause disease in humans. This type of virus participants.7 is the most frequently used in gene therapy.1 • Phase 4: A phase of research to describe clinical trials • Adenovirus: A member of a family of viruses that can cause occurring after FDA has approved a drug for marketing. infections in the respiratory tract, eye, and gastrointestinal They include post market requirement and commitment tract. studies that are required of or agreed to by the study • Adeno-Associated Virus Vector: Adeno viruses used as sponsor. These trials gather additional information about a vehicles for genes, whose core genetic material has been drug’s safety, efficacy, or optimal use.8 removed and replaced by the FVIII- or FIX-gene • Codon: a sequence of three nucleotides in DNA or RNA • Amino Acids: building block of a protein that gives instructions to add a specific amino acid to an • Antibody: a protein produced by immune cells called B-cells elongating protein in response to a foreign molecule; acts by binding to the • CRISPR: a family of DNA sequences that can be cleaved by molecule and often making it inactive or targeting it for specific enzymes, and therefore serve as a guide to cut out destruction and insert genes. -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Development of Sexual Dimorphism in the Drosophila Testis
review REVIEW Spermatogenesis 2:3, 129-136; July/August/September 2012; © 2012 Landes Bioscience Development of sexual dimorphism in the Drosophila testis Cale Whitworth, Erin Jimenez and Mark Van Doren* Department of Biology; The Johns Hopkins University; Baltimore, MD USA Keywords: Drosophila, gonad, germ cell, sexual dimorphism, testis, doublesex, DMRT, germline stem cell, stem cell niche The creation of sexual dimorphism in the gonads is essential for posterior (A/P) and dorsal/ventral (D/V) patterning systems that producing the male and female gametes required for sexual divide the mesoderm into distinct cell types (reviewed in ref. 1). reproduction. Sexual development of the gonads involves Three clusters of ≈12 SGPs each will form on either side of the both somatic cells and germ cells, which often undergo sex embryo in parasegments (PSs) 10–12 (ref. 2, Figure 1) (“paraseg- determination by different mechanisms. While many sex- specific characteristics evolve rapidly and are very different ments” are the units of segmental identity along the A/P axis). between animal species, gonad function and the formation Each mesodermal PS is divided into an anterior (“even skipped of sperm and eggs appear more similar and may be more (eve) domain”) and posterior (“sloppy paired domain”). SGPs conserved. Consistent with this, the doublesex/mab3 Related form within the eve domain while in other PSs this domain gives Transcription factors (DMRTs) are important for gonad sexual rise to the fat body.3,4 The D/V axis is also divided into distinct dimorphism in a wide range of animals, including flies, worms domains, and the SGPs in PS10–12 form within the dorso-lateral and mammals. -

Human Germline Genome Editing: Fact Sheet
Human germline genome editing: fact sheet Purpose • To contribute to evidence-informed discussions about human germline genome editing. KEY TAKEAWAYS • Gene editing offers the potential to improve human health in ways not previously possible. • Making changes to human genes that can be passed on to future generations is prohibited in Australia. • Unresolved questions remain on the possible long-term impacts, unintended consequences, and ethical issues associated with introducing heritable changes by editing of the genome of human gametes (sperm and eggs) and embryos. • AusBiotech believes the focus of human gene editing should remain on non-inheritable changes until such time as the scientific evidence, regulatory frameworks and health care models have progressed sufficiently to warrant consideration of any heritable genetic edits. Gene editing Gene editing is the insertion, deletion, or modification of DNA to modify an organism’s specific genetic characteristics. New and evolving gene editing techniques and tools (e.g. CRISPR) allow editing of genes with a level of precision that increases its applications across the health, agricultural, and industrial sectors. These breakthrough techniques potentially offer a range of different options for treating devastating human diseases and delivering environmentally sustainable food production systems that can feed the world’s growing population, which is expected to exceed nine billion by 2050. The current primary application of human gene editing is on non-reproductive cells (‘somatic’ cells) -

Neuron Morphology Influences Axon Initial Segment Plasticity
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 1-2016 Neuron Morphology Influences Axon Initial Segment Plasticity Allan T. Gulledge Dartmouth College, [email protected] Jaime J. Bravo Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Computational Neuroscience Commons, Molecular and Cellular Neuroscience Commons, and the Other Neuroscience and Neurobiology Commons Dartmouth Digital Commons Citation Gulledge, A.T. and Bravo, J.J. (2016) Neuron morphology influences axon initial segment plasticity, eNeuro doi:10.1523/ENEURO.0085-15.2016. This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. -

Satellite Glial Cell Communication in Dorsal Root Ganglia
Neuronal somatic ATP release triggers neuron– satellite glial cell communication in dorsal root ganglia X. Zhang, Y. Chen, C. Wang, and L.-Y. M. Huang* Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555-1069 Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved April 25, 2007 (received for review December 14, 2006) It has been generally assumed that the cell body (soma) of a release of ATP from the somata of DRG neurons. Here, we show neuron, which contains the nucleus, is mainly responsible for that electric stimulation elicits robust vesicular release of ATP from synthesis of macromolecules and has a limited role in cell-to-cell neuronal somata and thus triggers bidirectional communication communication. Using sniffer patch recordings, we show here that between neurons and satellite cells. electrical stimulation of dorsal root ganglion (DRG) neurons elicits robust vesicular ATP release from their somata. The rate of release Results events increases with the frequency of nerve stimulation; external We asked first whether vesicular release of ATP occurs in the -Ca2؉ entry is required for the release. FM1–43 photoconversion somata of DRG neurons. P2X2-EGFP receptors were overex analysis further reveals that small clear vesicles participate in pressed in HEK cells and used as the biosensor (i.e., sniffer patch exocytosis. In addition, the released ATP activates P2X7 receptors method) (20). P2X2 receptors were chosen because they were easily in satellite cells that enwrap each DRG neuron and triggers the expressed at a high level (Ϸ200 pA/pF) in HEK cells and the communication between neuronal somata and glial cells. -

Female and Male Gametogenesis 3 Nina Desai , Jennifer Ludgin , Rakesh Sharma , Raj Kumar Anirudh , and Ashok Agarwal
Female and Male Gametogenesis 3 Nina Desai , Jennifer Ludgin , Rakesh Sharma , Raj Kumar Anirudh , and Ashok Agarwal intimately part of the endocrine responsibility of the ovary. Introduction If there are no gametes, then hormone production is drastically curtailed. Depletion of oocytes implies depletion of the major Oogenesis is an area that has long been of interest in medicine, hormones of the ovary. In the male this is not the case. as well as biology, economics, sociology, and public policy. Androgen production will proceed normally without a single Almost four centuries ago, the English physician William spermatozoa in the testes. Harvey (1578–1657) wrote ex ovo omnia —“all that is alive This chapter presents basic aspects of human ovarian comes from the egg.” follicle growth, oogenesis, and some of the regulatory mech- During a women’s reproductive life span only 300–400 of anisms involved [ 1 ] , as well as some of the basic structural the nearly 1–2 million oocytes present in her ovaries at birth morphology of the testes and the process of development to are ovulated. The process of oogenesis begins with migra- obtain mature spermatozoa. tory primordial germ cells (PGCs). It results in the produc- tion of meiotically competent oocytes containing the correct genetic material, proteins, mRNA transcripts, and organ- Structure of the Ovary elles that are necessary to create a viable embryo. This is a tightly controlled process involving not only ovarian para- The ovary, which contains the germ cells, is the main repro- crine factors but also signaling from gonadotropins secreted ductive organ in the female. -

Sexual Reproduction: Meiosis, Germ Cells, and Fertilization 21
Chapter 21 Sexual Reproduction: Meiosis, Germ Cells, and Fertilization 21 Sex is not absolutely necessary. Single-celled organisms can reproduce by sim- In This Chapter ple mitotic division, and many plants propagate vegetatively by forming multi- cellular offshoots that later detach from the parent. Likewise, in the animal king- OVERVIEW OF SEXUAL 1269 dom, a solitary multicellular Hydra can produce offspring by budding (Figure REPRODUCTION 21–1), and sea anemones and marine worms can split into two half-organisms, each of which then regenerates its missing half. There are even some lizard MEIOSIS 1272 species that consist only of females that reproduce without mating. Although such asexual reproduction is simple and direct, it gives rise to offspring that are PRIMORDIAL GERM 1282 genetically identical to their parent. Sexual reproduction, by contrast, mixes the CELLS AND SEX DETERMINATION IN genomes from two individuals to produce offspring that differ genetically from MAMMALS one another and from both parents. This mode of reproduction apparently has great advantages, as the vast majority of plants and animals have adopted it. EGGS 1287 Even many procaryotes and eucaryotes that normally reproduce asexually engage in occasional bouts of genetic exchange, thereby producing offspring SPERM 1292 with new combinations of genes. This chapter describes the cellular machinery of sexual reproduction. Before discussing in detail how the machinery works, FERTILIZATION 1297 however, we will briefly consider what sexual reproduction involves and what its benefits might be. OVERVIEW OF SEXUAL REPRODUCTION Sexual reproduction occurs in diploid organisms, in which each cell contains two sets of chromosomes, one inherited from each parent.