Mummies from Puruchuco-Huaquerones, Peru
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Andean Textile Traditions: Material Knowledge and Culture, Part 1 Elena Phipps University of California, Los Angeles, [email protected]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln PreColumbian Textile Conference VII / Jornadas de Centre for Textile Research Textiles PreColombinos VII 11-13-2017 Andean Textile Traditions: Material Knowledge and Culture, Part 1 Elena Phipps University of California, Los Angeles, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/pct7 Part of the Art and Materials Conservation Commons, Chicana/o Studies Commons, Fiber, Textile, and Weaving Arts Commons, Indigenous Studies Commons, Latina/o Studies Commons, Museum Studies Commons, Other History of Art, Architecture, and Archaeology Commons, and the Other Languages, Societies, and Cultures Commons Phipps, Elena, "Andean Textile Traditions: Material Knowledge and Culture, Part 1" (2017). PreColumbian Textile Conference VII / Jornadas de Textiles PreColombinos VII. 10. http://digitalcommons.unl.edu/pct7/10 This Article is brought to you for free and open access by the Centre for Textile Research at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in PreColumbian Textile Conference VII / Jornadas de Textiles PreColombinos VII by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Andean Textile Traditions: Material Knowledge and Culture, Part 1 Elena Phipps In PreColumbian Textile Conference VII / Jornadas de Textiles PreColombinos VII, ed. Lena Bjerregaard and Ann Peters (Lincoln, NE: Zea Books, 2017), pp. 162–175 doi:10.13014/K2V40SCN Copyright © 2017 by the author. Compilation copyright © 2017 Centre for Textile Research, University of Copenhagen. 8 Andean Textile Traditions: Material Knowledge and Culture, Part 1 Elena Phipps Abstract The development of rich and complex Andean textile traditions spanned millennia, in concert with the development of cul- tures that utilized textiles as a primary form of expression and communication. -
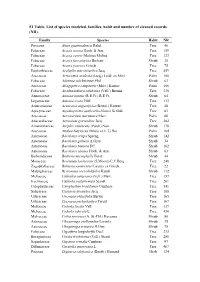
S1 Table. List of Species Modeled, Families, Habit and Number of Cleaned Records (NR)
S1 Table. List of species modeled, families, habit and number of cleaned records (NR). Family Species Habit NR Pinaceae Abies guatemalensis Rehd. Tree 46 Fabaceae Acacia aroma Hook. & Arn. Tree 159 Fabaceae Acacia caven (Molina) Molina Tree 123 Fabaceae Acacia furcatispina Burkart Shrub 35 Fabaceae Acacia praecox Griseb. Tree 75 Euphorbiaceae Acalypha macrostachya Jacq. Tree 459 Arecaceae Acrocomia aculeata (Jacq.) Lodd. ex Mart. Palm 166 Fabaceae Adesmia volckmannii Phil. Shrub 63 Arecaceae Allagoptera campestris (Mart.) Kuntze Palm 106 Fabaceae Anadenanthera colubrina (Vell.) Brenan Tree 330 Annonaceae Annona nutans (R.E.Fr.) R.E.Fr. Shrub 64 Loganiaceae Antonia ovata Pohl Tree 113 Araucariaceae Araucaria angustifolia (Bertol.) Kuntze Tree 48 Apocynaceae Aspidosperma quebracho-blanco Schltdl. Tree 83 Arecaceae Astrocaryum murumuru Mart. Palm 66 Anacardiaceae Astronium graveolens Jacq. Tree 282 Amaranthaceae Atriplex canescens (Pursh) Nutt. Shrub 178 Arecaceae Attalea butyracea (Mutis ex L.f.) We Palm 104 Asteraceae Baccharis crispa Spreng. Shrub 142 Asteraceae Baccharis gilliesii A.Gray Shrub 34 Asteraceae Baccharis trimera DC. Shrub 162 Asteraceae Baccharis ulicina Hook. & Arn. Shrub 63 Berberidaceae Berberis microphylla Forst. Shrub 44 Moraceae Brosimum lactescens (S.Moore) C.C.Berg Tree 248 Zygophyllaceae Bulnesia sarmientoi Lorentz ex Griseb. Tree 22 Malpighiaceae Byrsonima coccolobifolia Kunth Shrub 112 Meliaceae Cabralea canjerana (Vell.) Mart. Tree 157 Icacinaceae Calatola costaricensis Standl. Tree 201 Calophyllaceae Calophyllum brasiliense Cambess. Tree 541 Salicaceae Casearia decandra Jacq. Tree 188 Urticaceae Cecropia obtusifolia Bertol. Tree 165 Urticaceae Cecropia pachystachya Trécul Tree 167 Meliaceae Cedrela fissilis Vell. Tree 137 Meliaceae Cedrela odorata L. Tree 436 Malvaceae Ceiba speciosa (A. St.-Hil.) Ravenna Shrub 80 Asteraceae Chuquiraga avellanedae Lorentz Shrub 35 Asteraceae Chuquiraga erinacea D.Don Shrub 75 Fabaceae Copaifera langsdorffii Desf. -

Reflections and Observations on Peru's Past and Present Ernesto Silva Kennesaw State University, [email protected]
Journal of Global Initiatives: Policy, Pedagogy, Perspective Volume 7 Number 2 Pervuvian Trajectories of Sociocultural Article 13 Transformation December 2013 Epilogue: Reflections and Observations on Peru's Past and Present Ernesto Silva Kennesaw State University, [email protected] Follow this and additional works at: https://digitalcommons.kennesaw.edu/jgi Part of the International and Area Studies Commons, and the Social and Cultural Anthropology Commons This work is licensed under a Creative Commons Attribution 4.0 License. Recommended Citation Silva, Ernesto (2013) "Epilogue: Reflections and Observations on Peru's Past and Present," Journal of Global Initiatives: Policy, Pedagogy, Perspective: Vol. 7 : No. 2 , Article 13. Available at: https://digitalcommons.kennesaw.edu/jgi/vol7/iss2/13 This Article is brought to you for free and open access by DigitalCommons@Kennesaw State University. It has been accepted for inclusion in Journal of Global Initiatives: Policy, Pedagogy, Perspective by an authorized editor of DigitalCommons@Kennesaw State University. For more information, please contact [email protected]. Emesto Silva Journal of Global Initiatives Volume 7, umber 2, 2012, pp. l83-197 Epilogue: Reflections and Observations on Peru's Past and Present Ernesto Silva 1 The aim of this essay is to provide a panoramic socio-historical overview of Peru by focusing on two periods: before and after independence from Spain. The approach emphasizes two cultural phenomena: how the indigenous peo ple related to the Conquistadors in forging a new society, as well as how im migration, particularly to Lima, has shaped contemporary Peru. This contribu tion also aims at providing a bibliographical resource to those who would like to conduct research on Peru. -

Supplementary Materialsupplementary Material
10.1071/BT13149_AC © CSIRO 2013 Australian Journal of Botany 2013, 61(6), 436–445 SUPPLEMENTARY MATERIAL Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records Joseph T. MillerA,E, Daniel J. MurphyB, Simon Y. W. HoC, David J. CantrillB and David SeiglerD ACentre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600 Canberra, ACT 2601, Australia. BRoyal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia. CSchool of Biological Sciences, Edgeworth David Building, University of Sydney, Sydney, NSW 2006, Australia. DDepartment of Plant Biology, University of Illinois, Urbana, IL 61801, USA. ECorresponding author. Email: [email protected] Table S1 Materials used in the study Taxon Dataset Genbank Acacia abbreviata Maslin 2 3 JF420287 JF420065 JF420395 KC421289 KC796176 JF420499 Acacia adoxa Pedley 2 3 JF420044 AF523076 AF195716 AF195684; AF195703 Acacia ampliceps Maslin 1 KC421930 EU439994 EU811845 Acacia anceps DC. 2 3 JF420244 JF420350 JF419919 JF420130 JF420456 Acacia aneura F.Muell. ex Benth 2 3 JF420259 JF420036 JF420366 JF419935 JF420146 KF048140 Acacia aneura F.Muell. ex Benth. 1 2 3 JF420293 JF420402 KC421323 JQ248740 JF420505 Acacia baeuerlenii Maiden & R.T.Baker 2 3 JF420229 JQ248866 JF420336 JF419909 JF420115 JF420448 Acacia beckleri Tindale 2 3 JF420260 JF420037 JF420367 JF419936 JF420147 JF420473 Acacia cochlearis (Labill.) H.L.Wendl. 2 3 KC283897 KC200719 JQ943314 AF523156 KC284140 KC957934 Acacia cognata Domin 2 3 JF420246 JF420022 JF420352 JF419921 JF420132 JF420458 Acacia cultriformis A.Cunn. ex G.Don 2 3 JF420278 JF420056 JF420387 KC421263 KC796172 JF420494 Acacia cupularis Domin 2 3 JF420247 JF420023 JF420353 JF419922 JF420133 JF420459 Acacia dealbata Link 2 3 JF420269 JF420378 KC421251 KC955787 JF420485 Acacia dealbata Link 2 3 KC283375 KC200761 JQ942686 KC421315 KC284195 Acacia deanei (R.T.Baker) M.B.Welch, Coombs 2 3 JF420294 JF420403 KC421329 KC955795 & McGlynn JF420506 Acacia dempsteri F.Muell. -

Community Formation and the Emergence of the Inca
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2019 Assembling States: Community Formation And The meE rgence Of The ncI a Empire Thomas John Hardy University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the History of Art, Architecture, and Archaeology Commons Recommended Citation Hardy, Thomas John, "Assembling States: Community Formation And The meE rgence Of The ncaI Empire" (2019). Publicly Accessible Penn Dissertations. 3245. https://repository.upenn.edu/edissertations/3245 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/3245 For more information, please contact [email protected]. Assembling States: Community Formation And The meE rgence Of The Inca Empire Abstract This dissertation investigates the processes through which the Inca state emerged in the south-central Andes, ca. 1400 CE in Cusco, Peru, an area that was to become the political center of the largest indigenous empire in the Western hemisphere. Many approaches to this topic over the past several decades have framed state formation in a social evolutionary framework, a perspective that has come under increasing critique in recent years. I argue that theoretical attempts to overcome these problems have been ultimately confounded, and in order to resolve these contradictions, an ontological shift is needed. I adopt a relational perspective towards approaching the emergence of the Inca state – in particular, that of assemblage theory. Treating states and other complex social entities as assemblages means understanding them as open-ended and historically individuated phenomena, emerging from centuries or millennia of sociopolitical, cultural, and material engagements with the human and non-human world, and constituted over the longue durée. -

The Genus Anadenanthera in Amerindian Cultures
THE GENUS ANADENANTHERA IN AMERINDIAN CULTURES BY SIRI VON REIS ALTSCHUL, PH .D. RESEARCH FELLOW BOTANICAL MUSEUM HARVARD UNIVERSITY CAMBRIDGE, MASSACHUSETTS 1972 This monograph is dedicated in affectionate memory to the late DANIEL HERBERT EFRON, PH.D., M.D. 1913-1972 cherished friend of the author and of the Botanical Museum, a true scientist devoted to the interdisciplinary approach in the advancement of knowledge. A/""'f""'<J liz {i>U<// t~ },~u 0<<J4 ~ If;. r:J~ ~ //"'~uI ~ ()< d~ ~ !dtd't;:..1 "./.u.L .A Vdl0 If;;: ~ '" OU'''-k4 :/" tu-d ''''''"-''t2.. ?,,".jd,~ jft I;ft'- ?_rl; A~~ ~r'4tft,t -5 " q,.,<,4 ~~ l' #- /""/) -/~ "1'Ii;. ,1""", "/'/1'1",, I X C"'-r'fttt. #) (../..d ~;, . W,( ~ ~ f;r"'" y it;.,,J 11/" Y 4J.. %~~ l{jr~ t> ~~ ~txh '1'ix r 4 6~" c/<'T'''(''-;{' rn« ?.d ~;;1';;/ a-.d txZ-~ ~ ;o/~ <A.H-iz "" ~".,/( 1-/X< "..< ,:" -.... ~ ~ . JJlr-0? on . .it-(,0.1' r 4 -11<.1.- aw./{') -:JL. P7t;;"j~;1 S .d-At ;0~/lAQ<..t ,ti~?,f,.... vj "7rU<-'- ~I""" =iiR-I1;M~ a....k«<-l, ¢- f!!) d..;.:~ M ~ ~y£/1 ~/.u..-... It'--, "" # :Z:-,k. "i ~ "d/~ efL<.<~/ ,w 1'#,') /';~~;d-t a;.. tlArl-<7'" I .Ii;'~.1 (1(-;.,} >Lc -(l"7C),.,..,;.. :.... ,,:/ ~ /-V,~ , ,1" # (i F'"' l' fJ~~A- (.tG- ~/~ Z:--7Co- ,,:. ,L7r= f,-, , ~t) ahd-p;: fJ~ / tr>d .4 ~f- $. b".,,1 ~/. ~ pd. 1'7'-· X ~-t;;;;.,~;z jM ~0Y:tJ;; ~ """.,4? br;K,' ./.n.u" ~ 7r .".,.~,j~ ;;f;tT ~ ..4'./ ;pf,., tJd~ M_~ (./I<'/~.'. IU. et. c./,. ~L.y !f-t.<H>:t;.tu ~ ~,:,-,p., .....:. -

Reconsidering a Moche Site in Northern Peru
Tearing Down Old Walls in the New World: Reconsidering a Moche Site in Northern Peru Megan Proffitt The country of Peru is an interesting area, bordered by mountains on one side and the ocean on the other. This unique environment was home to numerous pre-Columbian cultures, several of which are well- known for their creativity and technological advancements. These cul- tures include such groups as the Chavin, Nasca, Inca, and Moche. The last of these, the Moche, flourished from about 0-800 AD and more or less dominated Peru’s northern coast. During the 2001 summer archaeological field season, I was granted the opportunity to travel to Peru and participate in the excavation of the Huaca de Huancaco, a Moche palace. In recent years, as Dr. Steve Bourget and his colleagues have conducted extensive research and fieldwork on the site, the cul- tural identity of its inhabitants have come into question. Although Huancaco has long been deemed a Moche site, Bourget claims that it is not. In this paper I will give a general, widely accepted description of the Moche culture and a brief history of the archaeological work that has been conducted on it. I will then discuss the site of Huancaco itself and my personal involvement with it. Finally, I will give a brief account of the data that have, and have not, been found there. This information is crucial for the necessary comparisons to other Moche sites required by Bourget’s claim that Huancaco is not a Moche site, a claim that will be explained and supported in this paper. -

The Evolution and Changes of Moche Textile Style: What Does Style Tell Us About Northern Textile Production?
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Textile Society of America Symposium Proceedings Textile Society of America 2002 The Evolution and Changes of Moche Textile Style: What Does Style Tell Us about Northern Textile Production? María Jesús Jiménez Díaz Universidad Complutense de Madrid Follow this and additional works at: https://digitalcommons.unl.edu/tsaconf Part of the Art and Design Commons Jiménez Díaz, María Jesús, "The Evolution and Changes of Moche Textile Style: What Does Style Tell Us about Northern Textile Production?" (2002). Textile Society of America Symposium Proceedings. 403. https://digitalcommons.unl.edu/tsaconf/403 This Article is brought to you for free and open access by the Textile Society of America at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Textile Society of America Symposium Proceedings by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. THE EVOLUTION AND CHANGES OF MOCHE TEXTILE STYLE: WHAT DOES STYLE TELL US ABOUT NORTHERN TEXTILE PRODUCTION? María Jesús Jiménez Díaz Museo de América de Madrid / Universidad Complutense de Madrid Although Moche textiles form part of the legacy of one of the best known cultures of pre-Hispanic Peru, today they remain relatively unknown1. Moche culture evolved in the northern valleys of the Peruvian coast (Fig. 1) during the first 800 years after Christ (Fig. 2). They were contemporary with other cultures such us Nazca or Lima and their textiles exhibited special features that are reflected in their textile production. Previous studies of Moche textiles have been carried out by authors such as Lila O'Neale (1946, 1947), O'Neale y Kroeber (1930), William Conklin (1978) or Heiko Pruemers (1995). -

ESJOA Spring 2011
Volume 6 Issue 1 C.S.U.D.H. ELECTRONIC STUDENT JOURNAL OF ANTHROPOLOGY Spring 2011 V O L U M E 6 ( 1 ) : S P R I N G 2 0 1 1 California State University Dominguez Hills Electronic Student Journal of Anthropology Editor In Chief Review Staff Scott Bigney Celso Jaquez Jessica Williams Maggie Slater Alex Salazar 2004 CSU Dominguez Hills Anthropology Club 1000 E Victoria Street, Carson CA 90747 Phone 310.243.3514 • Email [email protected] I Table of Contents THEORY CORNER Essay: Functionalism in Anthropological Theory By: Julie Wennstrom pp. 1-6 Abstract: Franz Boas, “Methods of Ethnology” By: Maggie Slater pp. 7 Abstract: Marvin Harris “Anthropology and the Theoretical and Paradigmatic Significance of the Collapse of Soviet and East European Communism By: Samantha Glover pp. 8 Abstract: Eleanor Burke Leacock “Women’s Status In Egalitarian Society: Implications For Social Evolution” By: Jessica Williams pp. 9 STUDENT RESEARCH Chinchorro Culture By: Kassie Sugimoto pp. 10-22 Reconstructing Ritual Change at Preceramic Asana By: Dylan Myers pp. 23-33 The Kogi (Kaggaba) of the Sierra Nevada de Santa Marta and the Kotosh Religious Tradition: Ethnographic Analysis of Religious Specialists and Religious Architecture of a Contemporary Indigenous Culture and Comparison to Three Preceramic Central Andean Highland Sites By: Celso Jaquez pp. 34-59 The Early Formative in Ecuador: The Curious Site of Real Alto By: Ana Cuellar pp. 60-70 II Ecstatic Shamanism or Canonist Religious Ideology? By: Samantha Glover pp. 71-83 Wari Plazas: An analysis of Proxemics and the Role of Public Ceremony By: Audrey Dollar pp. -

Languages of the Middle Andes in Areal-Typological Perspective: Emphasis on Quechuan and Aymaran
Languages of the Middle Andes in areal-typological perspective: Emphasis on Quechuan and Aymaran Willem F.H. Adelaar 1. Introduction1 Among the indigenous languages of the Andean region of Ecuador, Peru, Bolivia, northern Chile and northern Argentina, Quechuan and Aymaran have traditionally occupied a dominant position. Both Quechuan and Aymaran are language families of several million speakers each. Quechuan consists of a conglomerate of geo- graphically defined varieties, traditionally referred to as Quechua “dialects”, not- withstanding the fact that mutual intelligibility is often lacking. Present-day Ayma- ran consists of two distinct languages that are not normally referred to as “dialects”. The absence of a demonstrable genetic relationship between the Quechuan and Aymaran language families, accompanied by a lack of recognizable external gen- etic connections, suggests a long period of independent development, which may hark back to a period of incipient subsistence agriculture roughly dated between 8000 and 5000 BP (Torero 2002: 123–124), long before the Andean civilization at- tained its highest stages of complexity. Quechuan and Aymaran feature a great amount of detailed structural, phono- logical and lexical similarities and thus exemplify one of the most intriguing and intense cases of language contact to be found in the entire world. Often treated as a product of long-term convergence, the similarities between the Quechuan and Ay- maran families can best be understood as the result of an intense period of social and cultural intertwinement, which must have pre-dated the stage of the proto-lan- guages and was in turn followed by a protracted process of incidental and locally confined diffusion. -

BC2019-Printproginccovers-High.Pdf
CONTENTS Welcome with Acknowledgements 1 Talk Abstracts (Alphabetically by Presenter) 3 Programme (Friday) 32–36 Programme (Saturday) 37–41 Programme (Sunday) 42–46 Installations 47–52 Film Festival 53–59 Entertainment 67–68 Workshops 69–77 Visionary Art 78 Invited Speaker & Committee Biographies 79–91 University Map 93 Area Map 94 King William Court – Ground Floor Map 95 King William Court – Third Floor Map 96 Dreadnought Building Map (Telesterion, Underworld, Etc.) 97 The Team 99 Safer Spaces Policy 101 General Information 107 BREAK TIMES - ALL DAYS 11:00 – 11:30 Break 13:00 – 14:30 Lunch 16:30 – 17:00 Break WELCOME & ACKNOWLEDGEMENTS WELCOME & ACKNOWLEDGEMENTS for curating the visionary art exhibition, you bring that extra special element to BC. Ashleigh Murphy-Beiner & Ali Beiner for your hard work, in your already busy lives, as our sponsorship team, which gives us more financial freedom to put on such a unique event. Paul Callahan for curating the Psychedelic Cinema, a fantastic line up this year, and thanks to Sam Oliver for stepping in last minute to help with this, great work! Andy Millns for stepping up in programming our installations, thank you! Darren Springer for your contribution to the academic programme, your perspective always brings new light. Andy Roberts for your help with merchandising, and your enlightening presence. Julian Vayne, another enlightening and uplifting presence, thank you for your contribution! To Rob Dickins for producing the 8 circuit booklet for the welcome packs, and organising the book stall, your expertise is always valuable. To Maria Papaspyrou for bringing the sacred feminine and TRIPPth. -

John P. Harrington Papers 1907-1959
THE PAPERS OF John Peabody Harringtan IN THE Smithsonian Institution 1907-1957 VOLUME SEVEN A GUIDE TO THE FIELD NOTES: NATIVE AMERICAN HISTORY, LANGUAGE, AND CULTURE OF MEXICO/CENTRAL AMERICA/ SOUTH AMERICA I:DITRD Br Elaine L. Mills KRAUS INTER AJ 10 L Pl BLIC 110 Di ision of Kraus-Thom Jl )r 1lI1.allon LUl11tcd THE PAPERS OF John Peabody Harringtan IN THE Smithsonian Institution 1907-1957 VOLUME SEVEN A GUIDE TO THE FIELD NOTES: Native American History, Language, and Culture of Mexico/Central America/South America Prepared in the National Anthropological Archives Department ofAnthropology National Museum ofNatural History Washington, D.C. THE PAPERS OF John Peabody Harringtan IN THE Smithsonian Institution 1907-1957 VOLUME SEVEN A GUIDE TO THE FIELD NOTES: Native American History, Language, and Culture of Mexico/Central America/South America EDITED BY Elaine L. Mills KRAUS INTERNATIONAL PUBLICATIONS A Division of Kraus-Thomson Organization Limited White Plains, N.Y. © Copyright The Smithsonian Institution 1988 All rights reserved. No part ofthis work covered by the copyright hereon may be reproduced or used in any form or by any means-graphic, electronic, or mechanical, including photocopying, recording or taping, information storage and retrieval systems-without written permission ofthe publisher. First Printing Printed in the United States of America §TM The paper in this publication meets the minimum requirements of American National Standard for Information Science- Permanence of Papers for Contents Printed Library Materials, ANSI Z39.48-1984. Library ofCongress Cataloging-in-Publication Data INTRODUCTION VII / V1/l Harrington, John Peabody. Scope and Content ofthis Publication VII / vu The papers ofJohn Peabody Harrington in the Smithsonian Institution, 1907 -1957.