Evolution and Genetics of Precocious Burrowing Behavior in Peromyscus Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cross-Transmission Studies with Eimeria Arizonensis-Like Oocysts
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 6-1992 Cross-Transmission Studies with Eimeria arizonensis-like Oocysts (Apicomplexa) in New World Rodents of the Genera Baiomys, Neotoma, Onychomys, Peromyscus, and Reithrodontomys (Muridae) Steve J. Upton Kansas State University Chris T. McAllister Department of Veterans Affairs Medical Cente Dianne B. Brillhart Kansas State University Donald W. Duszynski University of New Mexico, [email protected] Constance D. Wash University of New Mexico Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Upton, Steve J.; McAllister, Chris T.; Brillhart, Dianne B.; Duszynski, Donald W.; and Wash, Constance D., "Cross-Transmission Studies with Eimeria arizonensis-like Oocysts (Apicomplexa) in New World Rodents of the Genera Baiomys, Neotoma, Onychomys, Peromyscus, and Reithrodontomys (Muridae)" (1992). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 184. https://digitalcommons.unl.edu/parasitologyfacpubs/184 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. J. Parasitol., 78(3), 1992, p. 406-413 ? American Society of Parasitologists 1992 CROSS-TRANSMISSIONSTUDIES WITH EIMERIAARIZONENSIS-LIKE OOCYSTS (APICOMPLEXA) IN NEWWORLD RODENTS OF THEGENERA BAIOMYS, NEOTOMA, ONYCHOMYS,PEROMYSCUS, AND REITHRODONTOMYS(MURIDAE) Steve J. Upton, Chris T. McAllister*,Dianne B. Brillhart,Donald W. Duszynskit, and Constance D. -

Special Publications Museum of Texas Tech University Number 63 18 September 2014
Special Publications Museum of Texas Tech University Number 63 18 September 2014 List of Recent Land Mammals of Mexico, 2014 José Ramírez-Pulido, Noé González-Ruiz, Alfred L. Gardner, and Joaquín Arroyo-Cabrales.0 Front cover: Image of the cover of Nova Plantarvm, Animalivm et Mineralivm Mexicanorvm Historia, by Francisci Hernández et al. (1651), which included the first list of the mammals found in Mexico. Cover image courtesy of the John Carter Brown Library at Brown University. SPECIAL PUBLICATIONS Museum of Texas Tech University Number 63 List of Recent Land Mammals of Mexico, 2014 JOSÉ RAMÍREZ-PULIDO, NOÉ GONZÁLEZ-RUIZ, ALFRED L. GARDNER, AND JOAQUÍN ARROYO-CABRALES Layout and Design: Lisa Bradley Cover Design: Image courtesy of the John Carter Brown Library at Brown University Production Editor: Lisa Bradley Copyright 2014, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (nsrl.ttu.edu). The authors and the Museum of Texas Tech University hereby grant permission to interested parties to download or print this publication for personal or educational (not for profit) use. Re-publication of any part of this paper in other works is not permitted without prior written permission of the Museum of Texas Tech University. This book was set in Times New Roman and printed on acid-free paper that meets the guidelines for per- manence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources. Printed: 18 September 2014 Library of Congress Cataloging-in-Publication Data Special Publications of the Museum of Texas Tech University, Number 63 Series Editor: Robert J. -
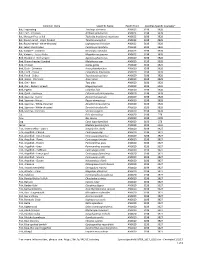
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

Multiple Captures of White-Footed Mice (Peromyscus Leucopus): Evidence for Social Structure? George A
Southern Illinois University Carbondale OpenSIUC Publications Department of Zoology 2008 Multiple Captures of White-footed Mice (Peromyscus leucopus): Evidence for Social Structure? George A. Feldhamer Southern Illinois University Carbondale Leslie B. Rodman Southern Illinois University Carbondale Timothy C. Carter Southern Illinois University Carbondale Eric M. Schauber Southern Illinois University Carbondale, [email protected] Follow this and additional works at: http://opensiuc.lib.siu.edu/zool_pubs Recommended Citation Feldhamer, George A., Rodman, Leslie B., Carter, Timothy C. and Schauber, Eric M. "Multiple Captures of White-footed Mice (Peromyscus leucopus): Evidence for Social Structure?." American Midland Naturalist 160 (Jan 2008): 171-177. doi:10.1674/ 0003-0031%282008%29160%5B171%3AMCOWMP%5D2.0.CO%3B2. This Article is brought to you for free and open access by the Department of Zoology at OpenSIUC. It has been accepted for inclusion in Publications by an authorized administrator of OpenSIUC. For more information, please contact [email protected]. Am. Midl. Nat. 160:171–177 Multiple Captures of White-footed Mice (Peromyscus leucopus): Evidence for Social Structure? GEORGE A. FELDHAMER,1 LESLIE B. RODMAN, 2 TIMOTHY C. CARTER AND ERIC M. SCHAUBER Department of Zoology, Southern Illinois University, Carbondale 62901 Cooperative Wildlife Research Laboratory, Southern Illinois University, Carbondale 62901 ABSTRACT.—Multiple captures (34 double, 6 triple) in standard Sherman live traps accounted for 6.3% of 1355 captures of Peromyscus leucopus (white-footed mice) in forested habitat in southern Illinois, from Oct. 2004 through Oct. 2005. There was a significant positive relationship between both the number and the proportion of multiple captures and estimated monthly population size. -

Nocturnal Rodents
Nocturnal Rodents Peter Holm Objectives (Chaetodipus spp. and Perognathus spp.) and The monitoring protocol handbook (Petryszyn kangaroo rats (Dipodomys spp.) belong to the 1995) states: “to document general trends in family Heteromyidae (heteromyids), while the nocturnal rodent population size on an annual white-throated woodrats (Neotoma albigula), basis across a representative sample of habitat Arizona cotton rat (Sigmodon arizonae), cactus types present in the monument”. mouse (Peromyscus eremicus), and grasshopper mouse (Onychomys torridus), belong to the family Introduction Muridae. Sigmodon arizonae, a native riparian Nocturnal rodents constitute the prey base for species relatively new to OPCNM, has been many snakes, owls, and carnivorous mammals. recorded at the Dos Lomitas and Salsola EMP All nocturnal rodents, except for the grasshopper sites, adjacent to Mexican agricultural fields. mouse, are primary consumers. Whereas Botta’s pocket gopher (Thomomys bottae) is the heteromyids constitute an important guild lone representative of the family Geomyidae. See of granivores, murids feed primarily on fruit Petryszyn and Russ (1996), Hoffmeister (1986), and foliage. Rodents are also responsible for Petterson (1999), Rosen (2000), and references considerable excavation and mixing of soil layers therein, for a thorough review. (bioturbation), “predation” on plants and seeds, as well as the dispersal and caching of plant seeds. As part of the Sensitive Ecosystems Project, Petryszyn and Russ (1996) conducted a baseline Rodents are common in all monument habitats, study originally titled, Special Status Mammals are easily captured and identified, have small of Organ Pipe Cactus National Monument. They home ranges, have high fecundity, and respond surveyed for nocturnal rodents and other quickly to changes in primary productivity and mammals in various habitats throughout the disturbance (Petryszyn 1995, Petryszyn and Russ monument and found that murids dominated 1996, Petterson 1999). -

Micheal L. Dent Richard R. Fay Arthur N. Popper Editors Rodent Bioacoustics Springer Handbook of Auditory Research
Springer Handbook of Auditory Research Micheal L. Dent Richard R. Fay Arthur N. Popper Editors Rodent Bioacoustics Springer Handbook of Auditory Research Volume 67 Series Editor Richard R. Fay, Ph.D., Loyola University Chicago, Chicago, IL, USA Arthur N. Popper, Ph.D., University of Maryland, College Park, MD, USA Editorial Board Karen Avraham, Ph.D., Tel Aviv University, Israel Andrew Bass, Ph.D., Cornell University Lisa Cunningham, Ph.D., National Institutes of Health Bernd Fritzsch, Ph.D., University of Iowa Andrew Groves, Ph.D., Baylor University Ronna Hertzano, M.D., Ph.D., School of Medicine, University of Maryland Colleen Le Prell, Ph.D., University of Texas, Dallas Ruth Litovsky, Ph.D., University of Wisconsin Paul Manis, Ph.D., University of North Carolina Geoffrey Manley, Ph.D., University of Oldenburg, Germany Brian Moore, Ph.D., Cambridge University, UK Andrea Simmons, Ph.D., Brown University William Yost, Ph.D., Arizona State University More information about this series at http://www.springer.com/series/2506 The ASA Press The ASA Press imprint represents a collaboration between the Acoustical Society of America and Springer dedicated to encouraging the publication of important new books in acoustics. Published titles are intended to reflect the full range of research in acoustics. ASA Press books can include all types of books published by Springer and may appear in any appropriate Springer book series. Editorial Board Mark F. Hamilton (Chair), University of Texas at Austin James Cottingham, Coe College Diana Deutsch, University of California, San Diego Timothy F. Duda, Woods Hole Oceanographic Institution Robin Glosemeyer Petrone, Threshold Acoustics William M. -

Reproduction, Growth and Development in Two Contiguously Allopatric Rodent Species, Genus Scotinomys
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 15 1 Reproduction, Growth and Development in Two Contiguously Allopatric Rodent Species, Genus Scotinomys by Emmet T. Hooper & Michael D. Carleton Ann Arbor MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN August 27, 1976 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN FRANCIS C. EVANS. EDITOR The publications of the Museum of Zoology, University of Michigan, consist of two series-the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 19 13, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number of pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mailing list for the series. The Miscellaneous Publications, which include papers on field and museum techniques, monographic studies, and other contributions not within the scope of the Occasional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zoology, Ann Arbor, Michigan 48109. MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN, NO. 15 1 Reproduction, Growth and Development in Two Contiguously Allopatric Rodent Species, Genus Scotinomys by Emmet T. -

SOCIAL ORGANIZATION of a SPECIES of SINGING MOUSE, Scotinomys Xerampelinus
SOCIAL ORGANIZATION OF A SPECIES OF SINGING MOUSE, Scotinomys xerampelinus By DIMITRI VINCENT BLONDEL A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2006 Copyright 2006 by Dimitri Vincent Blondel This thesis is dedicated to my parents, Pierre and Linda, who have supported me in all of my dreams and ambitions throughout my life. ACKNOWLEDGMENTS I would like to thank my committee members, Dr. Steven M. Phelps, Dr. H. Jane Brockmann, and Dr. Lyn Branch, for all of their help with this project. Dr. Brockmann and Dr. Phelps were co-chairs of my committee, and provided extremely valuable advice and support. Dr. Phelps introduced me to singing mice, the wonderful country of Panama, and ceviche, and provided generous funding and equipment support. Jorge Pino, our research assistant in the field, contributed greatly to this project. Dr. Rafael Samudio provided much appreciated logistical support with permits and other field arrangements in Panama. Thanks go to Dr. Jerry Wolff for providing radio-tracking training in Memphis, and for advice and discussion. Thanks go to Dr. Alex Ophir for help with statistics and graphs. Thanks go to Dr. Donald Dewsbury for sharing his knowledge of Scotinomys. The graduate students and post-doctoral associates in the Department of Zoology provided valued input; this includes Polly Campbell, Ondi Crino, Billy Gunnels, Joanna Matos, Toshi Okuyama, and many others. Thanks go to Dr. Mel Sunquist and Dr. Stephen Coates for providing my initial radio-tracking and live-trapping training in their field techniques course at the Ordway Preserve. -

List of 28 Orders, 129 Families, 598 Genera and 1121 Species in Mammal Images Library 31 December 2013
What the American Society of Mammalogists has in the images library LIST OF 28 ORDERS, 129 FAMILIES, 598 GENERA AND 1121 SPECIES IN MAMMAL IMAGES LIBRARY 31 DECEMBER 2013 AFROSORICIDA (5 genera, 5 species) – golden moles and tenrecs CHRYSOCHLORIDAE - golden moles Chrysospalax villosus - Rough-haired Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus – Lowland Streaked Tenrec 3. Microgale dobsoni - Dobson’s Shrew Tenrec 4. Tenrec ecaudatus – Tailless Tenrec ARTIODACTYLA (83 genera, 142 species) – paraxonic (mostly even-toed) ungulates ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BOVIDAE (46 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Impala 3. Alcelaphus buselaphus - Hartebeest 4. Alcelaphus caama – Red Hartebeest 5. Ammotragus lervia - Barbary Sheep 6. Antidorcas marsupialis - Springbok 7. Antilope cervicapra – Blackbuck 8. Beatragus hunter – Hunter’s Hartebeest 9. Bison bison - American Bison 10. Bison bonasus - European Bison 11. Bos frontalis - Gaur 12. Bos javanicus - Banteng 13. Bos taurus -Auroch 14. Boselaphus tragocamelus - Nilgai 15. Bubalus bubalis - Water Buffalo 16. Bubalus depressicornis - Anoa 17. Bubalus quarlesi - Mountain Anoa 18. Budorcas taxicolor - Takin 19. Capra caucasica - Tur 20. Capra falconeri - Markhor 21. Capra hircus - Goat 22. Capra nubiana – Nubian Ibex 23. Capra pyrenaica – Spanish Ibex 24. Capricornis crispus – Japanese Serow 25. Cephalophus jentinki - Jentink's Duiker 26. Cephalophus natalensis – Red Duiker 1 What the American Society of Mammalogists has in the images library 27. Cephalophus niger – Black Duiker 28. Cephalophus rufilatus – Red-flanked Duiker 29. Cephalophus silvicultor - Yellow-backed Duiker 30. Cephalophus zebra - Zebra Duiker 31. Connochaetes gnou - Black Wildebeest 32. Connochaetes taurinus - Blue Wildebeest 33. Damaliscus korrigum – Topi 34. -

Food Habits of Peromyscus and Reithrodontomys in the Wichita Mountains Wildlife Refuge, Oklahoma Anthony J
45 Food Habits of Peromyscus and Reithrodontomys in the Wichita Mountains Wildlife Refuge, Oklahoma Anthony J. Stancampiano¹ and William Caire Department of Biology, University of Central Oklahoma, Edmond, OK 73034 Received: 1994 May 26; Revised: 1995 Feb 14 Food habits of three sympatric species of Peromyscus (P. attwateri, P. leucopus, P. maniculatus) and Reithrodontomys fulvescens were studied in the Wichita Mountains Wildlife Refuge in Comanche County, Oklahoma, from March through October 1988, to determine if significant dietary overlap occurred among these species. Stomach contents were examined and identified microscopically. Coefficients of total dietary overlap between species of Peromyscus were all significant. Dietary overlap coefficients between the three species of Peromyscus and Reithrodontomys were not significant. Diets of P. attwateri, P. leucopus and P. maniculatus consisted of 45.8%, 41.7% and 50% plant matter and 54.2%, 58.3% and 50% animal matter, respectively. The diet of P. fulvescens consisted of 40% plant matter and 60% animal matter. Due to the extent of dietary overlap among species of Peromyscus, competition for food resources does not appear to be intense during the study period. INTRODUCTION Many studies of the trophic relations among small mammals have been conducted in the last three decades and many have focused on microtine rodents (1, 2). The few recent studies on non-microtine rodent food habits have been geographically scattered (3-8). In these studies, food items of individual species were listed, but few compared the extent of dietary overlap among members of a genus in a particular community (e.g., 3-5). This study examined the degree of dietary overlap (9) in food habits of Peromyscus attwateri, P. -

Phylogenetic Relationships in Neotomine-Peromyscine Rodents (Muroidea) and a Reappraisal of the Dichotomy Within New World Cricetinae
MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 157 Phylogenetic Relationships in Neotomine-Peromyscine Rodents (Muroidea) and a Reappraisal of the Dichotomy within New World Cricetinae by Michael Dean Carleton National Museum of Natural History Smithsonian Institution Washington, D.C. 20560 Ann Arbor MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN December 12, 1980 MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN The publications of the Museum of Zoology, University of Michigan, consist of two series - the Occasional Papers and the Miscellaneous Publications. Both series were founded by Dr. Bryant Walker, Mr. Bradshaw H. Swales, and Dr. W. W. Newcomb. The Occasional Papers, publication of which was begun in 1913, serve as a medium for original studies based principally upon the collections in the Museum. They are issued separately. When a sufficient number bf pages has been printed to make a volume, a title page, table of contents, and an index are supplied to libraries and individuals on the mail- ing list for the series. The Miscellaneous Publications. which include .DaDers . on field and museum tech- niques, monographic studies, and other contributions not within the scope of the Occa- sional Papers, are published separately. It is not intended that they be grouped into volumes. Each number has a title page and, when necessary, a table of contents. A complete list of publications on Birds, Fishes, Insects, Mammals, Mollusks, and Reptiles and Amphibians is available. Address inquiries to the Director, Museum of Zool- ogy, Ann Arbor, Michigan 48109. MISCELLANEOUS PUBLICATIONS MUSEUM OF ZOOLOGY, UNIVERSITY OF MICHIGAN NO. 157 Phylogenetic Relationships in Neotomine-Peromyscine Rodents (Muroidea) and a Reappraisal of the Dichotomy within New World Cricetinae hy Michael Dean Carleton National Museum of Natural History Smithsonian Institution Washington, D.C. -

Rodents of the Colorado River Corridor and Their Relationship with Campers
Rodents of the Colorado River corridor and their relationship with campers by Jessica Dettman There are seventeen rodent species known to exist in the Colorado River corridor of the Grand Canyon (Dettman 2005), although only some of these rodents regularly interact with humans. Members of the Peromyscus genus are probably the most commonly seen rodent species in the canyon, because they frequently seek out scraps leftover by campers and hunt for food in camp kitchens. Near the river, where most rafters camp, the most common species are the deer mouse (Peromyscus maniculatus), the canyon mouse (Peromyscus crinitus), and the cactus mouse (Peromyscus eremicus) (Carothers and Brown 1991). These species are often quite bold, and will come close to humans in order to gain access to food scraps in and around the camp. Peromyscus species, like many rodents, are primarily nocturnal. Therefore, they are most frequently seen from the early evening through the early morning, or from dusk to sunrise. During our early spring rafting trip down the canyon we saw only one Peromyscus individual. This individual was likely either a brush mouse P. boylii (Fig. 1) or canyon mouse P. crinitus. The mouse appeared just as the sun was setting, and approached the camp kitchen area. It was moving in and out of the rocky cliff area surrounding the kitchen, and allowed us to come within about one foot of it. It appeared to be pregnant although it could also have been overweight. We considered pregnancy to be the more likely explanation because March is a suitable pregnancy time for Peromyscus and an overweight individual following the winter months would be unlikely.