ICD-9-CM Official Guidelines for Coding and Reporting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Presence of ROS in Inflammatory Environment of Peri-Implantitis Tissue
Journal of Clinical Medicine Article Presence of ROS in Inflammatory Environment of Peri-Implantitis Tissue: In Vitro and In Vivo Human Evidence 1, 2, 2, 3 4 Eitan Mijiritsky y, Letizia Ferroni y, Chiara Gardin y, Oren Peleg , Alper Gultekin , Alper Saglanmak 4, Lucia Gemma Delogu 5, Dinko Mitrecic 6, Adriano Piattelli 7, 8, 2,9, , Marco Tatullo z and Barbara Zavan * z 1 Head and Neck Maxillofacial Surgery, Department of Otoryngology, Tel-Aviv Sourasky Medical Center, Sackler Faculty of Medicine, Tel-Aviv University, Weizmann street 6, 6423906 Tel-Aviv, Israel; [email protected] 2 Maria Cecilia Hospital, GVM Care & Research, Cotignola, 48033 Ravenna, Italy; [email protected] (L.F.); [email protected] (C.G.) 3 Department of Otolaryngology Head and Neck Surgery and Maxillofacial Surgery, Tel-Aviv Sourasky Medical Center, Sackler School of Medicine, Tel Aviv University, 701990 Tel-Aviv, Israel; [email protected] 4 Istanbul University, Faculty of Dentistry, Department of Oral Implantology, 34093 Istanbul, Turkey; [email protected] (A.G.); [email protected] (A.S.) 5 University of Padova, DpT Biomedical Sciences, 35133 Padova, Italy; [email protected] 6 School of Medicine, Croatian Institute for Brain Research, University of Zagreb, Šalata 12, 10 000 Zagreb, Croatia; [email protected] 7 Department of Medical, Oral, and Biotechnological Sciences, University of Chieti-Pescara, via dei Vestini 31, 66100 Chieti, Italy; [email protected] 8 Marrelli Health-Tecnologica Research Institute, Biomedical Section, Street E. Fermi, 88900 Crotone, Italy; [email protected] 9 Department of Medical Sciences, University of Ferrara, via Fossato di Mortara 70, 44123 Ferara, Italy * Correspondence: [email protected] Co-first author. -

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

SURGICAL INSTRUMENTS Veterinarians Are the Doctors Specializing in the Health of Animals
SURGICAL INSTRUMENTS Veterinarians are the doctors specializing in the health of animals. They do the necessary surgical operations and care for the well-being of the animal creatures. The very basic thing they need in a certain operation and care are the veterinary instruments. This will serve as the main allay of every veterinarian in providing care. (1) What are surgical instruments? Surgical instruments are essentially gadgets planned in an uncommon manner to perform particular capacities amid a surgical operation to improve viability and accomplishment of the surgery. (1) 4 Basic types of surgical instruments Surgical instruments are specially designed tools that assist health care professionals car- ry out specific actions during an operation. Most instruments crafted from the early 19th century on are made from durable stainless steel. Some are designed for general use, and others for spe- cific procedures. There are many surgical instruments available for almost any specialization in medicine. There are precision instruments used in microsurgery, ophthalmology and otology. Most surgical instruments can be classified into these 4 basic types: Cutting and Dissecting – these instruments usually have sharp edges or tips to cut through skin, tissue and suture material. Surgeons need to cut and dissect tissue to explore irregular growths and to remove dangerous or damaged tissue. These instruments have single or double razor- sharp edges or blades. Nurses need to be very careful to avoid injuries, and regularly inspect these instruments before using, for re-sharpening or replacement. 11 Iris Scissors 2016 – 1 – LV01-KA202 – 022652 This project is funded by the European Union Clamping and Occluding – are used in many surgical procedures for compressing blood vessels or hollow organs, to prevent their contents from leaking. -

Overview of Biomaterials and Their Use in Medical Devices
© 2003 ASM International. All Rights Reserved. www.asminternational.org Handbook of Materials for Medical Devices (#06974G) CHAPTER 1 Overview of Biomaterials and Their Use in Medical Devices A BIOMATERIAL, as defined in this hand- shapes, have relatively low cost, and be readily book, is any synthetic material that is used to available. replace or restore function to a body tissue and Figure 1 lists the various material require- is continuously or intermittently in contact with ments that must be met for successful total joint body fluids (Ref 1). This definition is somewhat replacement. The ideal material or material restrictive, because it excludes materials used combination should exhibit the following prop- for devices such as surgical or dental instru- erties: ments. Although these instruments are exposed A biocompatible chemical composition to to body fluids, they do not replace or augment • avoid adverse tissue reactions the function of human tissue. It should be noted, Excellent resistance to degradation (e.g., cor- however, that materials for surgical instru- • rosion resistance for metals or resistance to ments, particularly stainless steels, are reviewed biological degradation in polymers) briefly in Chapter 3, “Metallic Materials,” in Acceptable strength to sustain cyclic loading this handbook. Similarly, stainless steels and • endured by the joint shape memory alloys used for dental/endodon- A low modulus to minimize bone resorption tic instruments are discussed in Chapter 10, • High wear resistance to minimize wear- “Biomaterials for Dental Applications.” • debris generation Also excluded from the aforementioned defi- nition are materials that are used for external prostheses, such as artificial limbs or devices Uses for Biomaterials (Ref 3) such as hearing aids. -

Unicompartmental Knee Replacement
This is a repository copy of Unicompartmental Knee Replacement. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/120113/ Version: Accepted Version Article: Takahashi, T, Pandit, HG orcid.org/0000-0001-7392-8561 and Phil, D (2017) Unicompartmental Knee Replacement. Journal of Arthroscopy and Joint Surgery, 4 (2). pp. 55-60. ISSN 0021-8790 https://doi.org/10.1016/j.jajs.2017.08.009 © 2017 International Society for Knowledge for Surgeons on Arthroscopy and Arthroplasty. Published by Elsevier, a division of RELX India, Pvt. Ltd. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Accepted Manuscript Title: Unicompartmental Knee Replacement Author: Tsuneari Takahashi PII: S2214-9635(17)30041-X DOI: http://dx.doi.org/doi:10.1016/j.jajs.2017.08.009 Reference: JAJS 97 To appear in: Authors: Hemant G. -

Pictures of Central Venous Catheters
Pictures of Central Venous Catheters Below are examples of central venous catheters. This is not an all inclusive list of either type of catheter or type of access device. Tunneled Central Venous Catheters. Tunneled catheters are passed under the skin to a separate exit point. This helps stabilize them making them useful for long term therapy. They can have one or more lumens. Power Hickman® Multi-lumen Hickman® or Groshong® Tunneled Central Broviac® Long-Term Tunneled Central Venous Catheter Dialysis Catheters Venous Catheter © 2013 C. R. Bard, Inc. Used with permission. Bard, are trademarks and/or registered trademarks of C. R. Bard, Inc. Implanted Ports. Inplanted ports are also tunneled under the skin. The port itself is placed under the skin and accessed as needed. When not accessed, they only need an occasional flush but otherwise do not require care. They can be multilumen as well. They are also useful for long term therapy. ` Single lumen PowerPort® Vue Implantable Port Titanium Dome Port Dual lumen SlimPort® Dual-lumen RosenblattTM Implantable Port © 2013 C. R. Bard, Inc. Used with permission. Bard, are trademarks and/or registered trademarks of C. R. Bard, Inc. Non-tunneled Central Venous Catheters. Non-tunneled catheters are used for short term therapy and in emergent situations. MAHURKARTM Elite Dialysis Catheter Image provided courtesy of Covidien. MAHURKAR is a trademark of Sakharam D. Mahurkar, MD. © Covidien. All rights reserved. Peripherally Inserted Central Catheters. A “PICC” is inserted in a large peripheral vein, such as the cephalic or basilic vein, and then advanced until the tip rests in the distal superior vena cava or cavoatrial junction. -

Breast Cancer Screening HEDIS Tip Sheet
HEDIS® Tip Sheet Effectiveness of Care Measure Breast Cancer Screening Breast cancer is the most common type of cancer, and the second leading cause of cancer-related deaths among women in the United States. Approximately 237,000 cases of breast cancer are diagnosed in women, and about 41,000 women die each year of breast cancer.1 Mammography is an effective screening tool for early detection of breast cancer and reduction of breast cancer mortality. California Health & Wellness want to help your practice increase Healthcare Effectiveness Data and Information Set (HEDIS®) rates. This tip sheet outlines key details of the Breast Cancer Screening (BCS) measure, its codes and guidance for documentation. Measure Women ages 50–74 who had a mammogram to screen for breast cancer in the past two years.2 Exclusions: • Patients who meet the following – A unilateral mastectomy criteria anytime during the without a modifier and measurement year: a left mastectomy with – Medicare patients ages 66 and service dates 14 days or older enrolled in an institutional more apart. special needs plans (I-SNP) or – A unilateral mastectomy living long-term in an institution. without a modifier and a – Patients ages 66 and older with right mastectomy with service frailty and advanced illness. dates 14 days or more apart. – Patients in hospice. – Absence of the left breast and absence of the right breast on the • Patients with bilateral mastectomy. same or different dates of service. Any of the following meet the criteria – Both of the following (on the same for bilateral mastectomy: or different dates of service): – Bilateral mastectomy or history. -

Breast Reconstruction with Expanders and Implants
Evidence-Based Clinical Practice Guideline: Breast Reconstruction with Expanders and Implants INTRODUCTION Disclaimer Evidence-based guidelines are strategies for patient management, The American Cancer Society estimates that nearly 230,000 American developed to assist physicians in clinical decision making. This women were diagnosed with invasive breast cancer in 2011.1 Many of guideline was developed through a comprehensive review of the these individuals will require mastectomy and total reconstruction of scientific literature and consideration of relevant clinical experience, the breast. The diagnosis and subsequent process can create signifi- and describes a range of generally acceptable approaches to diagnosis, cant confusion and distress for the affected persons and their families management, or prevention of specific diseases or conditions. This and, consequently, surgical treatment and reconstructive procedures guideline attempts to define principles of practice that should are of utmost importance in the breast cancer care continuum. In generally meet the needs of most patients in most circumstances. 2011, the American Society of Plastic Surgeons® (ASPS) reported an increase in the rate of breast reconstructions, citing nearly 100,000 However, this guideline should not be construed as a rule, nor procedures, of which the majority employed expanders/implants.2 should it be deemed inclusive of all proper methods of care The 3% increase in reconstructions over the course of just one year or exclusive of other methods of care reasonably directed at highlights the significance of maintaining patient safety and obtaining the appropriate results. It is anticipated that it will be optimizing surgical outcomes. necessary to approach some patients’ needs in different ways. -

Information for Patients Having a Sigmoid Colectomy
Patient information – Pre-operative Assessment Clinic Information for patients having a sigmoid colectomy This leaflet will explain what will happen when you come to the hospital for your operation. It is important that you understand what to expect and feel able to take an active role in your treatment. Your surgeon will have already discussed your treatment with you and will give advice about what to do when you get home. What is a sigmoid colectomy? This operation involves removing the sigmoid colon, which lies on the left side of your abdominal cavity (tummy). We would then normally join the remaining left colon to the top of the rectum (the ‘storage’ organ of the bowel). The lines on the attached diagram show the piece of bowel being removed. This operation is done with you asleep (general anaesthetic). The operation not only removes the bowel containing the tumour but also removes the draining lymph glands from this part of the bowel. This is sent to the pathologists who will then analyse each bit of the bowel and the lymph glands in detail under the microscope. This operation can often be completed in a ‘keyhole’ manner, which means less trauma to the abdominal muscles, as the biggest wound is the one to remove the bowel from the abdomen. Sometimes, this is not possible, in which case the same operation is done through a bigger incision in the abdominal wall – this is called an ‘open’ operation. It does take longer to recover with an open operation but, if it is necessary, it is the safest thing to do. -

Renovascular Hypertension
z RENOGRAM INIS-mf —11322 RENOVASCULAR HYPERTENSION G.G. Geyskes STELLINGEN Behorende bij het proefschrift THE RENOGRAM IN RENOVASCULAR HYPERTENSION door G.G. Geyskes \ "n. it f 1 VII Het captopril renogratn kan de diagnostische PTA zoals door Maxwell Paranormale geneeskunde bij patiënten met hypertensie heeft meer bepleit vrijwel vervangen. subjectieve dan objectieve effecten. M.H. Maxwell. A.V. Walu. Hyptritnsion 1914.6:589-392. II VIII Bij dubbelzijdige nierarteriestenose geeft ncfrectomie van een kleine Het lijkt mogelijk de ontwikkeling van diabetische nefropathie af te nier in combinatie met PTA van de contralaterale nier vaak verbetering remmen door medicamenteuze antihypertensieve therapie, speciaal met van de totale nierfunktie. converting enzyme inhibitors. Ill IX Onderdrukking van de PRA is een antihyperteniief mechanisme van Het roken van sigaretten kan een oorzaak van renovaculaire hyperten- betaMokkers. sie zijn. G.G. Geyskts. J. Vos. P. Boer. E.J. Dorhoul Mets. Lmcei 1976:1049-1051. CE. Grimm el al. Nrphron 1986. 44 SI: 96-100. IV Contractie van het extracellulair volume is een antihyperteniief mecha- Het metaiodobenzylguanidine scintigram is een aanwinst bij de diag- nisme van diuretka. nostiek van het feochromocytoom. De opname van deze stof kan ook U.A.M. van Seht». G.G. Geyskti. J.C. Hom. El Dorhoul Mees. therapeutische consequenties hebben. CKn Phorm è Vier 1986. 39:6044, XI Bij veroordeling voor een /.waar misdrijf kan men 't best een levenslange Het clonidine withdrawal syndroom is een vrij sterke contraindicatie tegen het gebruik van dit antihyptttensivum. strul geven die verkort wordt bij verbetering van de mentaliteit. G.G. Geyskts. P. Boer. E.J. -

The Care of a Patient with Fournier's Gangrene
CASE REPORT The care of a patient with Fournier’s gangrene Esma Özşaker, Asst. Prof.,1 Meryem Yavuz, Prof.,1 Yasemin Altınbaş, MSc.,1 Burçak Şahin Köze, MSc.,1 Birgül Nurülke, MSc.2 1Department of Surgical Nursing, Ege University Faculty of Nursing, Izmir; 2Department of Urology, Ege University Faculty of Medicine Hospital, Izmir ABSTRACT Fournier’s gangrene is a rare, necrotizing fasciitis of the genitals and perineum caused by a mixture of aerobic and anaerobic microor- ganisms. This infection leads to complications including multiple organ failure and death. Due to the aggressive nature of this condition, early diagnosis is crucial. Treatment involves extensive soft tissue debridement and broad-spectrum antibiotics. Despite appropriate therapy, mortality is high. This case report aimed to present nursing approaches towards an elderly male patient referred to the urology service with a diagnosis of Fournier’s gangrene. Key words: Case report; Fournier’s gangrene; nursing diagnosis; patient care. INTRODUCTION Rarely observed in the peritoneum, genital and perianal re- perineal and genital regions, it is observed in a majority of gions, necrotizing fasciitis is named as Fournier’s gangrene.[1-5] cases with general symptoms, such as fever related infection It is an important disease, following an extremely insidious and weakness, and without symptoms in the perineal region, beginning and causing necrosis of the scrotum and penis by negatively influencing the prognosis by causing a delay in diag- advancing rapidly within one-two days.[1] The rate of mortal- nosis and treatment.[2,3] Consequently, anamnesis and physical ity in the literature is between 4 and 75%[6] and it has been examination are extremely important. -
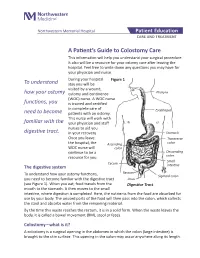
A Patient's Guide to Colostomy Care
Northwestern Memorial Hospital Patient Education CARE AND TREATMENT A Patient’s Guide to Colostomy Care This information will help you understand your surgical procedure. It also will be a resource for your ostomy care after leaving the hospital. Feel free to write down any questions you may have for your physician and nurse. During your hospital Figure 1 To understand stay you will be visited by a wound, how your ostomy ostomy and continence Pharynx (WOC) nurse. A WOC nurse functions, you is trained and certified in complete care of Esophagus need to become patients with an ostomy. This nurse will work with familiar with the your physician and staff nurses to aid you digestive tract. in your recovery. Stomach Once you leave Transverse the hospital, the Ascending colon WOC nurse will colon continue to be a Descending resource for you. colon Small Cecum The digestive system intestine Rectum To understand how your ostomy functions, Sigmoid colon you need to become familiar with the digestive tract Anus (see Figure 1). When you eat, food travels from the Digestive Tract mouth to the stomach. It then moves to the small intestine, where digestion is completed. Here, the nutrients from the food are absorbed for use by your body. The unused parts of the food will then pass into the colon, which collects the stool and absorbs water from the remaining material. By the time this waste reaches the rectum, it is in a solid form. When the waste leaves the body, it is called a bowel movement (BM), stool or feces.