Drug-Drug Interactions in Inpatient and Outpatient Settings in Iran: A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -

Expression of Multiple Populations of Nicotinic Acetylcholine
EXPRESSION OF MULTIPLE POPULATIONS OF NICOTINIC ACETYLCHOLINE RECEPTORS IN BOVINE ADRENAL CHROMAFFIN CELLS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By Bryan W. Wenger, B.A. The Ohio State University 2003 Dissertation Committee: Approved by: Professor Dennis B. McKay, Advisor Professor R. Thomas Boyd ________________________ Advisor Professor Popat N. Patil College of Pharmacy Professor Lane Wallace, Ph.D. ABSTRACT The importance of the role of nAChRs in physiological and pathological states is becoming increasingly clear. It is apparent that there are multitudes of nAChR subtypes with different expression patterns, pharmacologies and functions that may be important in various disease states. Therefore, a greater understanding of nAChR subtypes is essential for potential pharmacological intervention in nAChR systems. Bovine adrenal chromaffin cells are a primary culture of a neuronal type cell that express ganglionic types of nAChRs whose activation can be related to a functional response. While much is known about the outcome of functional activation of adrenal nAChRs, little work has been done in characterizing populations of nAChRs in adrenal chromaffin cells. These studies characterize the pharmacology and regulation of populations of nAChRs found in bovine adrenal chromaffin cells. The primary findings of this research include 1) the characterization of an irreversible antagonist of adrenal nAChRs, 2) the discovery of -

Muscle Relaxants Physiologic and Pharmacologic Aspects
K. Fukushima • R. Ochiai (Eds.) Muscle Relaxants Physiologic and Pharmacologic Aspects With 125 Figures Springer Contents Preface V List of Contributors XVII 1. History of Muscle Relaxants Some Early Approaches to Relaxation in the United Kingdom J.P. Payne 3 The Final Steps Leading to the Anesthetic Use of Muscle Relaxants F.F. Foldes 8 History of Muscle Relaxants in Japan K. Iwatsuki 13 2. The Neuromuscular Junctions - Update Mechanisms of Action of Reversal Agents W.C. Bowman 19 Nicotinic Receptors F.G. Standaert 31 The Neuromuscular Junction—Basic Receptor Pharmacology J.A. Jeevendra Martyn 37 Muscle Contraction and Calcium Ion M. Endo 48 3. Current Basic Experimental Works Related to Neuromuscular Blockade in Present and Future Prejunctional Actions of Neuromuscular Blocking Drugs I.G. Marshall, C. Prior, J. Dempster, and L. Tian 51 VII VIII Approaches to Short-Acting Neuromuscular Blocking Agents J.B. Stenlake 62 Effects Other than Relaxation of Non-Depolarizing Muscle Relaxants E.S. Vizi 67 Regulation of Innervation-Related Properties of Cultured Skeletal Muscle Cells by Transmitter and Co-Transmitters R.H. Henning 82 4. Current Clinical Experimental Works Related to Neuromuscular Blockade in Present and Future Where Should Experimental Works Be Conducted? R.D. Miller 93 Muscle Relaxants in the Intensive Care Unit ! J.E. Caldwell 95 New Relaxants in the Operating Room R.K. Mirakhur 105 Kinetic-Dynamic Modelling of Neuromuscular Blockade C. Shanks Ill 5. Basic Aspects of Neuromuscular Junction Physiology of the Neuromuscular Junction W.C. Bowman 117 Properties of <x7-Containing Acetylcholine Receptors and Their Expression in Both Neurons and Muscle D.K. -

Muscle Relaxants Femoral Arteriography.,,>
IN THIS ISSUE: , .:t.i ". Muscle Relaxants fI, \'.' \., Femoral ArteriographY.,,> University of Minnesota Medical Bulletin Editor ROBERT B. HOWARD, M.D. Associate Editors RAY M. AMBERG GILBERT S. CAMPBELL, M.D. ELLIS S. BENSON, MD. BYRON B. COCHRANE, M.D. E. B. BROWN, PhD. RICHARD T. SMITH, M.D. WESLEY W. SPINK, MD. University of Minnesota Medical School J. 1. MORRILL, President, University of Minnesota HAROLD S. DIEHL, MD., Dean, College of Medical SciencBs WILLIAM F. MALONEY, M.D., Assistant Dean N. 1. GAULT, JR., MD., Assistant Dean University Hospitals RAY M. AMBERG, Director Minnesota Medical Foundation WESLEY W. SPINK, M.D., President R. S. YLVISAKER, MD., Vice-President ROBERT B. HOWARD, M.D., Secretary-Treasurer Minnesota Medical Alumni Association BYRON B. COCHRANE, M.D., President VIRGIL J. P. LUNDQUIST, M.D., First Vice-President SHELDON M. LAGAARD, MD., Second Vice-President LEONARD A. BOROWICZ, M.D., Secretary JAMES C. MANKEY, M.D., Treasurer UNIVERSITY OF MINNESOTA Medical Bulletin OFFICIAL PUBLICATION OF THE UNIVERSITY OF MINNESOTA HOSPITALS, MINNE· SOTA MEDICAL FOUNDATION, AND MINNESOTA MEDICAL ALUMNI ASSOCIATION VOLUME XXVIII December 1, 1956 NUMBER 4 CONTENTS STAFF MEETING REPORTS Current Status of Muscle Relaxants BY J. Albert Jackson, MD., J. H. Matthews, M.D., J. J. Buckley, M.D., D.S.P. Weatherhead, M.D., AND F. H. Van Bergen, M.D. 114 Small Vessel Changes in Femoral Arteriography BY Alexander R. Margulis, M.D. AND T. O. Murphy, MD.__ 123 EDITORIALS .. - -. - ---__ __ _ 132 MEDICAL SCHOOL ACTIVITIES ----------- 133 POSTGRADUATE EDUCATION - 135 COMING EVENTS 136 PIIb1ished semi.monthly from October 15 to JUDe 15 at Minneapolis, Minnesota. -
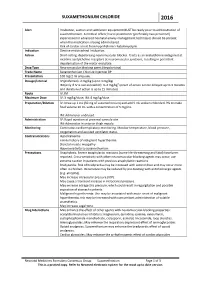
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

The Quest for the Origin and Prevention of Postoperative Myalgia Following Succinylcholine : New Insights in an Old Problem
The quest for the origin and prevention of postoperative myalgia following succinylcholine : new insights in an old problem Citation for published version (APA): Schreiber, J-U. (2010). The quest for the origin and prevention of postoperative myalgia following succinylcholine : new insights in an old problem. Datawyse / Universitaire Pers Maastricht. https://doi.org/10.26481/dis.20100702js Document status and date: Published: 01/01/2010 DOI: 10.26481/dis.20100702js Document Version: Publisher's PDF, also known as Version of record Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Pharmacological Studies on Pupillary Reflex Dilatation
PHARMACOLOGICAL STUDIES ON PUPILLARY REFLEX DILATATION SHINJI OONO Departmentof Pharmacology,Faculty of Medicine,Kyushu University, Fukuoka Received for publication September 20, 1964 Concerning the role of sympathetic and parasympathetic mechanisms in reflex dilatation of the pupil elicited by painful stimuli, there have long been many argu ments. One group of authors, for example, Bechterew (1) and Braunstein (2) concluded that the pupillary dilatation resulting from painful stimuli was caused solely by inhibi tion of the third cranial nerve activity. Another group of investigators, for example, Lieben and Kahn (3), Bain, Irving and McSwiney (4), Ury and Gellhorn (5), Ury and Oldberg (6) and Seybold and Moore (7) were of the opinion that parasympathetic in hibition was the principal factor in the reflex dilatation while sympathetic excitation was a negligible one. On the contrary, others, for instance, Luchsinger (8), Anderson (9), and Dechaume (10) claimed that sympathetic excitation was responsible for the pupillary reaction which was absent after cervical sympathectomy. Weinstein and Bender (11) compared the pupillary reflex activity in cats and mon keys. They concluded that a species-difference exists: in both species pupillary dilata tion is accomplished by both parasympathetic inhibitory and sympathetic excitatory mechanism. In the cat, inhibition of the parasympathetic mechanism is predominant while in the monkey excitation of the sympathetic mechanism is of greater importance . Later Lowenstein and Loewenfeld (12) performed more detailed experiments in cats using their own pupillographic instrument; they observed that pupillary reflex dilata tion was mostly due to sympathetic excitation, and was reduced to less than one-fifth of the normal control dilatation after sympathectomy. -

Doxacurium Chloride Injection Abbott Laboratories
NUROMAX - doxacurium chloride injection Abbott Laboratories ---------- NUROMAX® (doxacurium chloride) Injection This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. NOT FOR USE IN NEONATES CONTAINS BENZYL ALCOHOL DESCRIPTION NUROMAX (doxacurium chloride) is a long-acting, nondepolarizing skeletal muscle relaxant for intravenous administration. Doxacurium chloride is [1α,2β(1'S*,2'R*)]-2,2' -[(1,4-dioxo-1,4- butanediyl)bis(oxy-3,1-propanediyl)]bis[1,2,3,4-tetrahydro-6,7,8-trimethoxy-2- methyl-1-[(3,4,5- trimethoxyphenyl)methyl]isoquinolinium] dichloride (meso form). The molecular formula is C56H78CI2N2O16 and the molecular weight is 1106.14. The compound does not partition into the 1- octanol phase of a distilled water/ 1-octanol system, i.e., the n-octanol:water partition coefficient is 0. Doxacurium chloride is a mixture of three trans, trans stereoisomers, a dl pair [(1R,1'R ,2S,2'S ) and (1S,1'S ,2R,2'R )] and a meso form (1R,1'S,2S,2'R). The meso form is illustrated below: NUROMAX Injection is a sterile, nonpyrogenic aqueous solution (pH 3.9 to 5.0) containing doxacurium chloride equivalent to 1 mg/mL doxacurium in Water for Injection. Hydrochloric acid may have been added to adjust pH. NUROMAX Injection contains 0.9% w/v benzyl alcohol. Reference ID: 2867706 CLINICAL PHARMACOLOGY NUROMAX binds competitively to cholinergic receptors on the motor end-plate to antagonize the action of acetylcholine, resulting in a block of neuromuscular transmission. This action is antagonized by acetylcholinesterase inhibitors, such as neostigmine. Pharmacodynamics NUROMAX is approximately 2.5 to 3 times more potent than pancuronium and 10 to 12 times more potent than metocurine. -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

COMPARISON of ROCURONIUM BROMIDE and SUCCINYLCHOLINE CHLORIDE for USE DURING RAPID SEQUENCE INTUBATION in ADULTS Ch
DOI: 10.18410/jebmh/2015/672 ORIGINAL ARTICLE COMPARISON OF ROCURONIUM BROMIDE AND SUCCINYLCHOLINE CHLORIDE FOR USE DURING RAPID SEQUENCE INTUBATION IN ADULTS Ch. Penchalaiah1, N. Jagadeesh Babu2, T. Kumar3 HOW TO CITE THIS ARTICLE: Ch. Penchalaiah, N. Jagadeesh Babu, T. Kumar. ”Comparison of Rocuronium Bromide and Succinylcholine Chloride for Use during Rapid Sequence Intubation in Adults”. Journal of Evidence based Medicine and Healthcare; Volume 2, Issue 32, August 10, 2015; Page: 4796-4806, DOI: 10.18410/jebmh/2015/672 ABSTRACT: BACKGROUND AND OBJECTIVE: The goal of rapid sequence intubation is to secure the patients airway smoothly and quickly, minimizing the chances of regurgitation and aspiration of gastric contents. Traditionally succinylcholine chloride has been the neuromuscular blocking drug of choice for use in rapid sequence intubation because of its rapid onset of action and profound relaxation. Succinylcholine chloride remains unsurpassed in providing ideal intubating conditions. However the use of succinylcholine chloride is associated with many side effects like muscle pain, bradycardia, hyperkalaemia and rise in intragastric and intraocular pressure. Rocuronium bromide is the only drug currently available which has the rapidity of onset of action like succinylcholine chloride. Hence the present study was undertaken to compare rocuronium bromide with succinylcholine chloride for use during rapid sequence intubation in adult patients. METHODOLOGY: The study population consisted of 90 patients aged between 18-60 years posted for various elective surgeries requiring general anaesthesia. Study population was randomly divided into 3 groups with 30 patients in each sub group. 1. Group I: Intubated with 1 mg kg-1 of succinylcholine chloride (n=30). -

Neuronal Nicotinic Receptors
NEURONAL NICOTINIC RECEPTORS Dr Christopher G V Sharples and preparations lend themselves to physiological and pharmacological investigations, and there followed a Professor Susan Wonnacott period of intense study of the properties of nAChR- mediating transmission at these sites. nAChRs at the Department of Biology and Biochemistry, muscle endplate and in sympathetic ganglia could be University of Bath, Bath BA2 7AY, UK distinguished by their respective preferences for C10 and C6 polymethylene bistrimethylammonium Susan Wonnacott is Professor of compounds, notably decamethonium and Neuroscience and Christopher Sharples is a hexamethonium,5 providing the first hint of diversity post-doctoral research officer within the among nAChRs. Department of Biology and Biochemistry at Biochemical approaches to elucidate the structure the University of Bath. Their research and function of the nAChR protein in the 1970’s were focuses on understanding the molecular and facilitated by the abundance of nicotinic synapses cellular events underlying the effects of akin to the muscle endplate, in electric organs of the acute and chronic nicotinic receptor electric ray,Torpedo , and eel, Electrophorus . High stimulation. This is with the goal of affinity snakea -toxins, principallyaa -bungarotoxin ( - Bgt), enabled the nAChR protein to be purified, and elucidating the structure, function and subsequently resolved into 4 different subunits regulation of neuronal nicotinic receptors. designateda ,bg , and d .6 An additional subunit, e , was subsequently identified in adult muscle. In the early 1980’s, these subunits were cloned and sequenced, The nicotinic acetylcholine receptor (nAChR) arguably and the era of the molecular analysis of the nAChR has the longest history of experimental study of any commenced. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal