Fact Sheet Ocfentanil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Understanding and Challenging the Drugs: Chemistry and Toxicology
UNDERSTANDING AND CHALLENGING THE DRUGS: CHEMISTRY AND TOXICOLOGY Presenter: • Dr. Jasmine Drake, Graduate Program Director and Assistant Professor, Administration of Justice Department, Barbara Jordan-Mickey Leland School of Public Affairs, Texas Southern University NACDL Training Defending Drug Overdose Homicides in Pennsylvania Penn State Harrisburg, Middletown, PA November 6th, 2019 11:30- 12:45 p.m. Understanding & Challenging the Drugs: Chemistry & Toxicology Dr. Jasmine Drake, Forensic Science Learning Laboratory, Texas Southern University I. Opioid Drug Classifications A. Types of Opioids B. Classic vs. Synthetic C. Toxicology of Opioids 1) How opioids interact with the body 2) Addiction (psychological vs. physiological II. New Classes of Drugs A. Emerging Threats B. Potency III. National Trends in Opioid Overdose Deaths in the U.S. A. Based on State B. Ethnicity C. Drug-Type (prescription vs. fentanyl vs. heroin) IV. Trends of Opioid Overdose Deaths in Philadelphia A. Based on Ethnicity B. Drug Type (prescription vs. fentanyl vs. heroin) V. Legal Considerations to the Opioid Epidemic A. Punitive Measures vs. Rehabilitative Treatment B. Progressive Jurisdictions Nationwide C. New Legal Measures in Philadelphia VI. Toxicology Reports A. What’s in the report? B. Key Aspects of the Tox Report C. Terminology D. Evaluating and Interpreting the data? E. Questions and considerations. VII. Conclusion and Discussion A. Case Specific Examples B. Sample Toxicology Reports The Opioid Epidemic: What labs have to do with it? Ewa King, Ph.D. Associate Director of Health RIDOH State Health Laboratories Analysis. Answers. Action. www.aphl.org Overview • Overdose trends • Opioids and their effects • Analytical testing approaches • Toxicology laboratories Analysis. Answers. Action. -

UCSF UC San Francisco Previously Published Works
UCSF UC San Francisco Previously Published Works Title Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review Permalink https://escholarship.org/uc/item/8xh0s7nf Authors Armenian, Patil Vo, Kathy Barr-Walker, Jill et al. Publication Date 2017-10-01 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review Patil Armenian, Kathy Vo, Jill Barr-Walker, Kara Lynch University of California, San Francisco-Fresno and University of California, San Francisco Keywords: opioid, synthetic opioids, fentanyl, fentanyl analog, carfentanil, naloxone Abbreviations: 4-ANPP: 4-anilino-N-phenethyl-4-piperidine; ANPP 4Cl-iBF: 4-chloroisobutyryfentanyl 4F-iBF: 4-fluoroisobutyrfentanyl AEI: Advanced electronic information AMF: alpha-methylfentanyl CBP: US Customs and Border Protection CDC: Centers for Disease Control CDSA: Controlled Drug and Substance Act (Canada) CNS: central nervous system DEA: US Drug Enforcement Agency DTO: Drug trafficking organization ED: Emergency department ELISA: enzyme-linked immunosorbent assay EMCDDA: European Monitoring Centre for Drug and Drug Addiction FDA: US Food and Drug Administration GC-MS: gas chromatography mass spectrometry ICU: intensive care unit IN: intranasal IV: intravenous LC-HRMS: liquid chromatography high resolution mass spectrometry LC-MS/MS: liquid chromatography tandem mass spectrometry MDA: United Kingdom Misuse of Drugs Act NPF: non-pharmaceutical fentanyl THF-F: tetrahydrofuranfentanyl US: United States USPS: US Postal Service UNODC: United Nations office on drugs and crime 1. Introduction The death rate due to opioid analgesics nearly quadrupled in the US from 1999 to 2011 and was responsible for 33,091 deaths in 2015 (CDC, 2014; Rudd et al., 2016). -

Use of Fentanyl, Butyrfentanyl and Furanylfentanyl As Discussed on Polish Online Forums Devoted to ‘Designer Drugs’
Psychiatr. Pol. ONLINE FIRST Nr 219: 1–18 Published ahead of print 12 March 2021 www.psychiatriapolska.pl ISSN 0033-2674 (PRINT), ISSN 2391-5854 (ONLINE) DOI: https://doi.org/10.12740/PP/OnlineFirst/128156 Use of fentanyl, butyrfentanyl and furanylfentanyl as discussed on Polish online forums devoted to ‘designer drugs’ Monika Kacela, Jakub Wojcieszak, Jolanta B. Zawilska Medical University of Lodz, Department of Pharmacodynamics Summary Aim. The study was aimed to analyze information posted by users of synthetic opioids on Polish online drug discussion forums. Special emphasis was given to sources of drugs and their availability, routes of administration, dosages, expected and toxic effects. Material and methods. 6,143 reports related to synthetic opioids, posted between 2005 and mid 2019 on three widely available popular Polish online forums devoted to psychoactive substances, i.e., https://hyperreal.info/talk, https://dopek.info and https://forum.dopalamy, were collected and analyzed. The article presents data on three most popular opioids, i.e., fentanyl, butyrfentanyl and furanylfentanyl. Results. Fentanyl was the most widely used and relatively easily accessible synthetic opioid in Poland. Butyrfentanyl and furanylfentanyl were far less popular. The main source of fentanyl was diversion of medicines, notably transdermal patches. Fentanyl, butyrfentanyl and furanylfentanyl are administered orally, buccally, sublingually, intranasally, by inhalation and intravenously. Concomitant use of fentanyl and its derivatives with other psychoactive compounds increases the risk of severe adverse effects. Conclusions. Our study contributed to a more comprehensive understanding of problems related to abuse of fentanyl, butyrfentanyl and furanylfentanyl in Poland. In the light of the relatively high popularity of pharmaceutical fentanyl used for non-medical purposes, there is an urgent need for more strict control over illegal sales of fentanyl transdermal preparations via the Internet, as well as disposal of used patches. -

No Moral Panic: Public Health Responses to Illicit Fentanyls
No Moral Panic: Public Health Responses to Illicit Fentanyls Testimony of Daniel Ciccarone, M.D., M.P.H. Professor, Department of Family and Community Medicine, University of California San Francisco Before the House Committee on the Judiciary, Subcommittee on Crime, Terrorism and Homeland Security United States House of Representatives Hearing: Fentanyl Analogues: Perspectives on Class Scheduling January 28, 2020 Chair Bass, Vice-Chair Demings, Ranking Member Ratcliffe and other distinguished members of the House Subcommittee on Crime, Terrorism and Homeland Security, thank you very much for the opportunity to testify before you today. It is indeed an honor to be here. My name is Dan Ciccarone. I am professor of family and community medicine at the University of California, San Francisco. I have been a clinician for over 30 years and an academic researcher in the area of substance use with a focus on the medical and public health consequences of heroin use for the past 20 years. I have been asked to speak on my perspective on the class scheduling of fentanyl analogues and how these regulations might work counter to the goals of public health. My research and that of my team is multidisciplinary and multi-level. We use the tools of epidemiology, anthropology, statistics, economics, clinical and basic sciences. I am appreciative of my funder, which is the National Institutes of Health, National Institute on Drug Abuse, as well as my team, which includes Dr. Jay Unick, University of Maryland, Dr. Sarah G. Mars, UCSF, Dr. Dan Rosenblum, Dalhousie University, and Dr. Georgiy Bobashev from RTI, North Carolina. -

General Agreement on Tariffs Andtrade
RESTRICTED GENERAL AGREEMENT TAR/W/87/Rev.1 16 June 1994 ON TARIFFS AND TRADE Limited Distribution (94-1266) Committee on Tariff Concessions HARMONIZED COMMODITY DESCRIPTION AND CODING SYSTEM (Harmonized System) Classification of INN Substances Revision The following communication has been received from the Nomenclature and Classification Directorate of the Customs Co-operation Council in Brussels. On 25 May 1993, we sent you a list of the INN substances whose classification had been discussed and decided by the Harmonized System Committee. At the time, we informed you that the classification of two substances, clobenoside and meclofenoxate, would be decided later. Furthermore, for some of the chemicals given in that list, one of the contracting parties had entered a reservation and the Harmonized System Committee therefore reconsidered its earlier decision in those cases. I am therefore sending you herewith a revised complete list of the classification decisions of the INN substances. In this revised list, two substances have been added and the classifications of two have been revised as explained below: (a) Addition Classification of clobenoside, (subheading 2940.00) and meclofenoxate (subheading 2922.19). (b) Amendment Etafedrine and moxidentin have now been reclassified in subheadings 2939.40 and 2932.29 respectively. The list of INN substances reproduced hereafter is available only in English. TAR/W/87/Rev. 1 Page 2 Classification of INN Substances Agreed by the Harmonized System Committee in April 1993 Revision Description HS Code -

OCFENTANIL Critical Review Report Agenda Item 4.5
OCFENTANIL Critical Review Report Agenda Item 4.5 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 39th ECDD (2017) Agenda item 4.5 Ocfentanil Page 2 of 17 39th ECDD (2017) Agenda item 4.5 Ocfentanil Contents Acknowledgements .......................................................................................................................... 5 Summary .......................................................................................................................................... 6 1. Substance identification ........................................................................................................... 7 A. International non-proprietary name (INN) ...................................................................................... 7 B. Chemical Abstract Service (CAS) registry number .......................................................................... 7 C. Other names ..................................................................................................................................... 7 D. Trade names ..................................................................................................................................... 7 E. Street names ..................................................................................................................................... 7 F. Physical properties ........................................................................................................................... 7 G. WHO review history ........................................................................................................................ -

16.19.20 Nmac 1 Title 16 Occupational And
TITLE 16 OCCUPATIONAL AND PROFESSIONAL LICENSING CHAPTER 19 PHARMACISTS PART 20 CONTROLLED SUBSTANCES 16.19.20.1 ISSUING AGENCY: Regulation and Licensing Department - Board of Pharmacy. [16.19.20.1 NMAC - Rp 16.19.20.1 NMAC, 06-26-2018] 16.19.20.2 SCOPE: All persons or entities that manufacture, distribute, dispense, administer, prescribe, deliver, analyze, or conduct research using controlled substances. [16.19.20.2 NMAC - Rp 16.19.20.2 NMAC, 06-26-2018] 16.19.20.3 STATUTORY AUTHORITY: Section 30-31-11 of the Controlled Substances Act, 30-31-1 through 30-31-42 NMSA 1978, authorizes the board of pharmacy to promulgate regulations and charge reasonable fees for the registration and control of the manufacture, distribution and dispensing of controlled substances. [16.19.20.3 NMAC - Rp 16.19.20.3 NMAC, 06-26-2018] 16.19.20.4 DURATION: Permanent. [16.19.20.4 NMAC - Rp 16.19.20.4 NMAC, 06-26-2018] 16.19.20.5 EFFECTIVE DATE: June 26, 2018, unless a different date is cited at the end of a section. [16.19.20.5 NMAC - Rp 16.19.20.5 NMAC, 06-26-2018] 16.19.20.6 OBJECTIVE: The objective of Part 20 of Chapter 19 is to protect the public health and welfare of the citizens of New Mexico by controlling and monitoring access to controlled substances and to give notice of the board’s designation of particular substances as controlled substances. [16.19.20.6 NMAC - Rp 16.19.20.6 NMAC, 06-26-2018] 16.19.20.7 DEFINITIONS: [RESERVED] [16.19.20.7 NMAC - Rp 16.19.20.7 NMAC, 06-26-2018] 16.19.20.8 REGISTRATION REQUIREMENTS: Persons required to register: A. -

The International Drug Control Conventions
ST/CND/1/Add.1/Rev.3 The International Drug Control Conventions Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol, as at 22 April 2017 UNITED NATIONS New York, 2017 ST/CND/1/Add.1/Rev.3 © United Nations, 2017. All rights reserved, worldwide. Schedules of the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol, as at 22 April 2017 List of drugs included in Schedule I Acetorphine 3-O-Acetyltetrahydro-7α-(1-hydroxy-1-methylbutyl)- 6,14-endo-ethenooripavine Acetyl-alpha-methylfentanyl N-[1-(α-Methylphenethyl)-4-piperidyl]acetanilide Acetylfentanyl N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]acetamide Acetylmethadol 3-Acetoxy-6-dimethylamino-4,4-diphenylheptane AH-7921 3,4-dichloro-N-{[1- (dimethylamino)cyclohexyl]methyl}benzamide Alfentanil N-[1-[2-(4-Ethyl-4,5-dihydro-5-oxo-1H-tetrazol-1-yl) ethyl]-4-(methoxymethyl)-4-piperidinyl]-N- phenylpropanamide Allylprodine 3-Allyl-1-methyl-4-phenyl-4-propionoxypiperidine Alphacetylmethadol α-3-Acetoxy-6-dimethylamino-4,4-diphenylheptane Alphameprodine α-3-Ethyl-1-methyl-4-phenyl-4-propionoxypiperidine Alphamethadol α-6-Dimethylamino-4,4-diphenyl-3-heptanol alpha-Methylfentanyl N-[1-(α-Methylphenethyl)-4-piperidyl]propionanilide alpha-Methylthiofentanyl N-[1-[1-Methyl-2-(2-thienyl)ethyl]-4-piperidyl] propionanilide Alphaprodine α-l,3-Dimethyl-4-phenyl-4-propionoxypiperidine Anileridine 1-p-Aminophenethyl-4-phenylpiperidine-4-carboxylic acid ethyl ester Benzethidine 1-(2-Benzyloxyethyl)-4-phenylpiperidine-4-carboxylic acid ethyl ester -

Fentanyl and Fentanyl Analogues Dear Judge Pryor
FEDERAL DEFENDER SENTENCING GUIDELINES COMMITTEE Lyric Office Centre 440 Louisiana Street, Suite 1350 Houston, Texas 77002-1634 Chair: Marjorie Meyers Phone: 713.718.4600 November 13, 2017 Honorable William H. Pryor, Jr. Acting Chair United States Sentencing Commission One Columbus Circle, N.E. Suite 2-500, South Lobby Washington, D.C. 20002-8002 Re: Public Comment on Fentanyl and Fentanyl Analogues Dear Judge Pryor: The Commission seeks comment on a number of issues related to fentanyl and fentanyl analogues. Because fentanyl and its analogues account for very few federal drug trafficking offenses,1 and unlike other drugs,2 there has been no reported litigation regarding the appropriate drug equivalency or whether a substance was a fentanyl analogue, most of the information the Commission receives during the comment period will not be derived from federal cases. Defenders have strongly encouraged the Commission to undertake a comprehensive review of the direct harms caused by particular doses of all drugs in the guidelines and amend the guidelines to create proportionate sentences. Such a comprehensive approach is necessary because focusing on a spotlighted drug like fentanyl and its analogues would only exacerbate the disproportionalities in §2D1.1. Much of the disparity created in the drug guidelines is a result of the Commission repeatedly increasing sentences for whatever drug is the current cause cèlebrè with no evidence that increased penalties reduce use or deter distribution.3 1 USSC, Quick Facts: Drug Trafficking Offenses (July 2017) (in FY 2016, crack cocaine, methamphetamine, powder, heroin, oxycodone, and marijuana accounted for 96.3% of drug trafficking offenses). 2 Most of the federal litigation regarding analogues or the appropriate drug quantity has involved synthetic cathinones, cannabinoids, and MDMA even though they account for fewer drug trafficking offenses than the six drugs discussed in the Quick Facts report). -
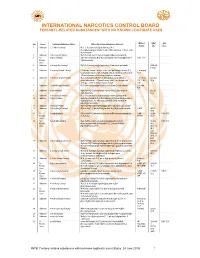
INTERNATIONAL NARCOTICS CONTROL BOARD FENTANYL-RELATED Substancesa with NO KNOWN LEGITIMATE USES
INTERNATIONAL NARCOTICS CONTROL BOARD a FENTANYL-RELATED SUBSTANCES WITH NO KNOWN LEGITIMATE USES Abbrev- CAS Intl No. Uses b Common Substance Name Other/ Alternative Substance Name(s) c iations No.d Ctrl.e 1 Unknown 2,2'-difluorofentanyl N-(1-(2-fluorophenethyl)piperidin-4-yl)-N-(2- fluorophenyl)propionamide; 2'-ortho-difluorofentanyl; 2'-fluoro ortho- fluorofentanyl 2 Unknown 2-fluoro butyrfentanyl N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4-yl) butyramide 3 No 2-fluorofentanyl ortho-fluorofentanyl; N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4- 2-FF; o-FF Known yl)propionamide Uses 4 Unknown 2-furanylethyl fentanyl N-[1-[2-(2-furanyl)ethyl]-4-piperidinyl]-N-phenyl-propanamide 1443-49- 8 (HCl) 5 Unknown 2-isopropylfuranyl fentanyl 2-isopropyl furanyl fentanyl; ortho-isopropyl furanyl fentanyl; N-(2- 2-isopropyl isopropylphenyl)-N-(1-phenethylpiperidin-4-yl)furan-2-carboxamide; Fu-F 2-Furanylfentanyl ortho-2-isopropylphenyl analogue 6 Unknown 2-methoxy furanyl fentanyl N-(2-methoxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2- 2-methoxy 101343- furancarboxamide; 2-Furanylfentanyl ortho-2-methoxyphenyl FuF; 2-Meo- 50-4 analogue; ortho-methoxy furanyl fentanyl FuF 7 Unknown 2-methyl furanyl fentanyl N-(1-phenethylpiperidin-4-yl)-N-(o-tolyl)furan-2-carboxamide 2-methyl FuF 8 Unknown 3-allyl fentanyl N-phenyl-N-[1-(2-phenylethyl)-3-(prop-2-en-1-yl)piperidin-4- 82208- yl]propanamide 84-2 9 Unknown 3-fluoro butyrfentanyl N-(3-fluorophenyl)-N-(1-phenethylpiperidin-4-yl) butyramide 10 Unknown 3-fluorofentanyl meta-fluorofentanyl; N-(3-fluorophenyl)-N-(1-phenethylpiperidin-4-