Bond Elut Certify Methods Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Understanding and Challenging the Drugs: Chemistry and Toxicology
UNDERSTANDING AND CHALLENGING THE DRUGS: CHEMISTRY AND TOXICOLOGY Presenter: • Dr. Jasmine Drake, Graduate Program Director and Assistant Professor, Administration of Justice Department, Barbara Jordan-Mickey Leland School of Public Affairs, Texas Southern University NACDL Training Defending Drug Overdose Homicides in Pennsylvania Penn State Harrisburg, Middletown, PA November 6th, 2019 11:30- 12:45 p.m. Understanding & Challenging the Drugs: Chemistry & Toxicology Dr. Jasmine Drake, Forensic Science Learning Laboratory, Texas Southern University I. Opioid Drug Classifications A. Types of Opioids B. Classic vs. Synthetic C. Toxicology of Opioids 1) How opioids interact with the body 2) Addiction (psychological vs. physiological II. New Classes of Drugs A. Emerging Threats B. Potency III. National Trends in Opioid Overdose Deaths in the U.S. A. Based on State B. Ethnicity C. Drug-Type (prescription vs. fentanyl vs. heroin) IV. Trends of Opioid Overdose Deaths in Philadelphia A. Based on Ethnicity B. Drug Type (prescription vs. fentanyl vs. heroin) V. Legal Considerations to the Opioid Epidemic A. Punitive Measures vs. Rehabilitative Treatment B. Progressive Jurisdictions Nationwide C. New Legal Measures in Philadelphia VI. Toxicology Reports A. What’s in the report? B. Key Aspects of the Tox Report C. Terminology D. Evaluating and Interpreting the data? E. Questions and considerations. VII. Conclusion and Discussion A. Case Specific Examples B. Sample Toxicology Reports The Opioid Epidemic: What labs have to do with it? Ewa King, Ph.D. Associate Director of Health RIDOH State Health Laboratories Analysis. Answers. Action. www.aphl.org Overview • Overdose trends • Opioids and their effects • Analytical testing approaches • Toxicology laboratories Analysis. Answers. Action. -

Medication Instructions for Allergy Patients
MEDICATION INSTRUCTIONS FOR ALLERGY PATIENTS Drugs which contain antihistamine or have antihistaminic effects can result in negative reactions to skin testing. As a result, it may not be possible to properly interpret skin test results, and testing may have to be repeated at a later date. While this list is extensive, it is NOT all inclusive (particularly of the various brand names). Discontinue ALL antihistamines including the following medications seven (7) days prior to skin testing (unless longer time specified): Antihistamines – Generic name (Brand name(s)): Cetirizine (Zyrtec, Zyrtec-D) Hydroxyzine (Vistaril, Atarax) Desloratadine (Clarinex) Levocetirizine (Xyzal) Fexofenadine (Allegra, Allegra-D) Loratadine (Claritin, Claritin-D, Alavert) Diphenhydramine (Aleve PM, Benadryl, Bayer P.M., Benylin, Contac P.M., Doans P.M, Excedrin PM, Legatrin P.M.. Nytol, Tylenol Nighttime, Unisom, Zzzquil) Chlorpheniramine (Aller-Chlor, Allerest, Alka Seltzer Plus, Chlor-Trimeton, Comtrex, Contac, Co-Pyronil, Coricidin, CTM, Deconamine, Dristan, Dura-tap, Naldecon, Ornade Spansules, Rondec, Sinutab, Teldrin, Triaminic, Triaminicin, Tylenol Allergy) Azatadine (Optimine, Trinalin) Doxylamine (Nyquil) Brompheniramine (Bromfed, Dimetane, Dimetapp) Meclizine (Antivert) Carbinoxamine (Clistin, Rondec) Pheniramine Clemastine (Tavist) Phenyltoloxamine (Nadecon) Cyclizine (Marezine) Promethazine (Phenergan) Cyprohepatidine (Periactin) (9 days) Pyrilamine (Mepyramine) Dexbrompheniramine (Drixoral) Quinacrine (Atabrine) Dexchlorpheniramine (Extendryl, Polaramine) -

WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/107 (2006.01) A61K 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A 61 47/10 (2006.0V) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/034361 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2012 (20.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/480,259 28 April 201 1 (28.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant (for all designated States except US): BOARD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, OF REGENTS, THE UNIVERSITY OF TEXAS SYS¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, TEM [US/US]; 201 West 7th St., Austin, TX 78701 (US). -

Mail Order Maintenance Medication Exclusion List
Maintenance Medication Exclusion List The following is a list of drugs that are excluded as maintenance medications. Medications which are listed here cannot be filled through the mail order pharmacy for the mail order incentive. This list includes both formulary/preferred and non-formulary/non-preferred medications, and does not provide information regarding the specific coverage, limitations, exclusions or quotas an individual member may have. Some dosage forms and strengths of a particular drug may be considered maintenance medications, whereas others may not. This list is sorted alphabetically by generic drug name. Generic drug names are written in lower case letters. Brand drug names (or some generic drugs with a trade name) are written in CAPITAL letters. Some drugs do not have a brand name available, in such cases the generic name is listed in the “Brand Name” column. This list of drugs should not be used to determine pharmacy benefits, such as prescription copay/coinsurance amounts or formulary status. If you have questions about the formulary status of a medication, or your prescription benefits, please call our Member Services Department at 1-888-681-7878 (toll free). For the hearing or speech impaired: 1-800-521-4874 (toll free TTY). The medications on this list are subject to change at any time. Exclusions: Any formulation that is required to be administered by a skilled medical professional or in a medical office, drugs infused in the home or in an infusion center, drugs that may have other quantity restrictions, any state law that may prohibit the mailing of certain dosage formulation of a drug, drugs that have a high potential for waste and diversion, drugs that require temperature control upon mailing and drugs that require refrigeration. -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Trends in Prescription Medication Use Among Children and Adolescents— United States, 1999-2014
Supplementary Online Content Hales CM, Kit BK, Gu Q, Ogden CL. Trends in prescription medication use among children and adolescents— United States, 1999-2014. JAMA. doi:10.1001/jama.2018.5690 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class This supplementary material was provided by the authors to give readers additional informtion about their work. © 2018 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/27/2021 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class Therapeutic classes are based on the Lexicon Plus prescription medication database and only those classes reported in the manuscript are listed. ADHD Medications Antiadrenergic Agents, Centrally Acting Clonidine Guanfacine CNS Stimulants Amphetamines Amphetamine Amphetamine; Dextroamphetamine Dextroamphetamine Lisdexamfetamine Methylphenidate or Dexmethylphenidate Dexmethylphenidate Methylphenidate Other CNS Stimulant Pemoline Selective Norepinephrine Reuptake Inhibitor Atomoxetine Antibiotics Cephalosporins Cefadroxil Cephalexin Cefaclor Cefprozil Cefuroxime Loracarbef Cefdinir Cefditoren Cefixime Cefpodoxime Ceftibuten Ceftriaxone Glycopeptide Antibiotics Vancomycin H. Pylori Eradication Agents Amoxicillin; Clarithromycin; Lansoprazole Lincomycin Derivatives Clindamycin Macrolide Derivatives Telithromycin Azithromycin Clarithromycin -

Fentanyl and Fentanyl Analogues Dear Judge Pryor
FEDERAL DEFENDER SENTENCING GUIDELINES COMMITTEE Lyric Office Centre 440 Louisiana Street, Suite 1350 Houston, Texas 77002-1634 Chair: Marjorie Meyers Phone: 713.718.4600 November 13, 2017 Honorable William H. Pryor, Jr. Acting Chair United States Sentencing Commission One Columbus Circle, N.E. Suite 2-500, South Lobby Washington, D.C. 20002-8002 Re: Public Comment on Fentanyl and Fentanyl Analogues Dear Judge Pryor: The Commission seeks comment on a number of issues related to fentanyl and fentanyl analogues. Because fentanyl and its analogues account for very few federal drug trafficking offenses,1 and unlike other drugs,2 there has been no reported litigation regarding the appropriate drug equivalency or whether a substance was a fentanyl analogue, most of the information the Commission receives during the comment period will not be derived from federal cases. Defenders have strongly encouraged the Commission to undertake a comprehensive review of the direct harms caused by particular doses of all drugs in the guidelines and amend the guidelines to create proportionate sentences. Such a comprehensive approach is necessary because focusing on a spotlighted drug like fentanyl and its analogues would only exacerbate the disproportionalities in §2D1.1. Much of the disparity created in the drug guidelines is a result of the Commission repeatedly increasing sentences for whatever drug is the current cause cèlebrè with no evidence that increased penalties reduce use or deter distribution.3 1 USSC, Quick Facts: Drug Trafficking Offenses (July 2017) (in FY 2016, crack cocaine, methamphetamine, powder, heroin, oxycodone, and marijuana accounted for 96.3% of drug trafficking offenses). 2 Most of the federal litigation regarding analogues or the appropriate drug quantity has involved synthetic cathinones, cannabinoids, and MDMA even though they account for fewer drug trafficking offenses than the six drugs discussed in the Quick Facts report). -
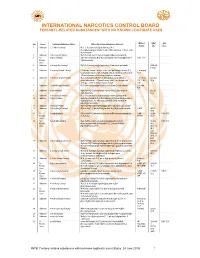
INTERNATIONAL NARCOTICS CONTROL BOARD FENTANYL-RELATED Substancesa with NO KNOWN LEGITIMATE USES
INTERNATIONAL NARCOTICS CONTROL BOARD a FENTANYL-RELATED SUBSTANCES WITH NO KNOWN LEGITIMATE USES Abbrev- CAS Intl No. Uses b Common Substance Name Other/ Alternative Substance Name(s) c iations No.d Ctrl.e 1 Unknown 2,2'-difluorofentanyl N-(1-(2-fluorophenethyl)piperidin-4-yl)-N-(2- fluorophenyl)propionamide; 2'-ortho-difluorofentanyl; 2'-fluoro ortho- fluorofentanyl 2 Unknown 2-fluoro butyrfentanyl N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4-yl) butyramide 3 No 2-fluorofentanyl ortho-fluorofentanyl; N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4- 2-FF; o-FF Known yl)propionamide Uses 4 Unknown 2-furanylethyl fentanyl N-[1-[2-(2-furanyl)ethyl]-4-piperidinyl]-N-phenyl-propanamide 1443-49- 8 (HCl) 5 Unknown 2-isopropylfuranyl fentanyl 2-isopropyl furanyl fentanyl; ortho-isopropyl furanyl fentanyl; N-(2- 2-isopropyl isopropylphenyl)-N-(1-phenethylpiperidin-4-yl)furan-2-carboxamide; Fu-F 2-Furanylfentanyl ortho-2-isopropylphenyl analogue 6 Unknown 2-methoxy furanyl fentanyl N-(2-methoxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2- 2-methoxy 101343- furancarboxamide; 2-Furanylfentanyl ortho-2-methoxyphenyl FuF; 2-Meo- 50-4 analogue; ortho-methoxy furanyl fentanyl FuF 7 Unknown 2-methyl furanyl fentanyl N-(1-phenethylpiperidin-4-yl)-N-(o-tolyl)furan-2-carboxamide 2-methyl FuF 8 Unknown 3-allyl fentanyl N-phenyl-N-[1-(2-phenylethyl)-3-(prop-2-en-1-yl)piperidin-4- 82208- yl]propanamide 84-2 9 Unknown 3-fluoro butyrfentanyl N-(3-fluorophenyl)-N-(1-phenethylpiperidin-4-yl) butyramide 10 Unknown 3-fluorofentanyl meta-fluorofentanyl; N-(3-fluorophenyl)-N-(1-phenethylpiperidin-4- -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Appendix-2Final.Pdf 663.7 KB
North West ‘Through the Gate Substance Misuse Services’ Drug Testing Project Appendix 2 – Analytical methodologies Overview Urine samples were analysed using three methodologies. The first methodology (General Screen) was designed to cover a wide range of analytes (drugs) and was used for all analytes other than the synthetic cannabinoid receptor agonists (SCRAs). The analyte coverage included a broad range of commonly prescribed drugs including over the counter medications, commonly misused drugs and metabolites of many of the compounds too. This approach provided a very powerful drug screening tool to investigate drug use/misuse before and whilst in prison. The second methodology (SCRA Screen) was specifically designed for SCRAs and targets only those compounds. This was a very sensitive methodology with a method capability of sub 100pg/ml for over 600 SCRAs and their metabolites. Both methodologies utilised full scan high resolution accurate mass LCMS technologies that allowed a non-targeted approach to data acquisition and the ability to retrospectively review data. The non-targeted approach to data acquisition effectively means that the analyte coverage of the data acquisition was unlimited. The only limiting factors were related to the chemical nature of the analyte being looked for. The analyte must extract in the sample preparation process; it must chromatograph and it must ionise under the conditions used by the mass spectrometer interface. The final limiting factor was presence in the data processing database. The subsequent study of negative MDT samples across the North West and London and the South East used a GCMS methodology for anabolic steroids in addition to the General and SCRA screens.