Quality Kinetics and Storage Stability Studies of Ready to Eat Peanut Chutney
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View Newsletter
0. ..........................................................................................................................................................................................7 1. Aloo Palak.................................................................................................................................................................7 2. Gobi Manchurian.....................................................................................................................................................7 3. Sindhi Saibhaji..........................................................................................................................................................8 4. Shahi Paneer .............................................................................................................................................................9 5. Potato in Curd Gravy.............................................................................................................................................10 6. Navratan Korma .....................................................................................................................................................11 7. Malai Kofta.............................................................................................................................................................12 8. Samosa.....................................................................................................................................................................13 -

South Indian Cuisine
SOUTH INDIAN CUISINE South Indian Cuisine is a term used to refer to the cuisines found in the four southern states of India, namely Andhra Pradesh, Karnataka, Kerala and Tamil Nadu. As opposed to North Indian cuisine, there is limited use of garam masala and other dried spices except cardamom, black pepper and turmeric. South Indian cuisine is rice based. Rice is combined with lentils to make wonderful dosas, idlis, vadas and uttapams. These items are glorious and delicious besides being nourishing and digestible (due to the fermenting process). They are combined with sambhar (dal), rasam (tamarind dal), dry and curried vegetable and pachadi (yogurt). Their rice preparations are also masterpieces like biryani from Hyderabad, lemon rice and rice seasoned with coconut peanuts, tamarind, chilies, curry leaves, urad dal and fenugreek seeds. South Indian chutneys are made of tamarind, coconut, peanuts, dal, fenugreek seeds, and cilantro. Meals are followed by coffee. South Indian dals and curries are more soupy than North Indian dals and curries. South Indian cuisine is also hotter. Coconut milk straight from the nut is a common beverage and sight in South India. Coffee is very popular in South India and Madras coffee is popular in South Indian restaurants throughout the world. The South Indian food is a brilliant blend of flavors, colors, seasoning, nutritional balance, fragrance, taste, and visual appeal. PARIMARAL - THE SOUTH INDIAN TRADITION OF SERVING A TRADITIONAL MEAL A typical traditional meal in South India is served on a "Vazhaillai", a freshly cut plantain leaf. The Sappad or food that is served on a banana leaf (even the size of the leaf varies from one community to another) is displayed like an identity card. -

Waiter.Com at for the Most Updated List of Restaurants
How to Order 1 Choose a restaurant from this delivery catalog according to your city or visit our website waiter.com at www.waiter.com/delivery for the most updated list of restaurants. The Web Site for Your Appetite! 2 Register online at www.waiter.com and order online using your UserName and Pass- word. (If you prefer to order by phone, please call us at 1-800-WAITER-9 to place your order.) To order online, just click on the desired restaurant’s menu items and click “checkout” when finishedordering to select the delivery date and time of your order. Why use Waiter.com? Too busy to pick up food for the office? Let 3 After placing your order, you will receive confirmation via email and by phone. Our Waiter.com do the work! We’re the best courteous delivery drivers will then deliver your meal at the time requested. Please choice for restaurant delivery and catering check your order items carefully as all sales are final after our delivery drivers leave. If for office meetings and events. Our clients there is a missing item, please call us at 1-800-WAITER-9 or 1-800-924-8379. Enjoy! include prominent and growing local com- panies, and we’d like to add you to our list Corporate Delivery Minimums* and Delivery Charges of satisfied corporate customers! Irving and North Dallas .....................................$35* minimum food order Delivery Charge per restaurant ..........................$6.95 Order Online or Call us! Driver Support Charge .......................................15% of order Online: www.waiter.com/delivery *Note: Delivery with NO minimum food order size is also available though a $5.00 small order Phone: 1-800-WAITER-9 surcharge will apply. -

5A5727d714063-1319853-Sample
Notion Press Old No. 38, New No. 6 McNichols Road, Chetpet Chennai - 600 031 First Published by Notion Press 2018 Copyright © Aparna Mudiganti Parinam 2018 All Rights Reserved. ISBN 978-1-948372-37-4 This book has been published with all reasonable efforts taken to make the material error-free after the consent of the author. No part of this book shall be used, reproduced in any manner whatsoever without written permission from the author, except in the case of brief quotations embodied in critical articles and reviews. The Author of this book is solely responsible and liable for its content including but not limited to the views, representations, descriptions, statements, information, opinions and references [“Content”]. The Content of this book shall not constitute or be construed or deemed to reflect the opinion or expression of the Publisher or Editor. Neither the Publisher nor Editor endorse or approve the Content of this book or guarantee the reliability, accuracy or completeness of the Content published herein and do not make any representations or warranties of any kind, express or implied, including but not limited to the implied warranties of merchantability, fitness for a particular purpose. The Publisher and Editor shall not be liable whatsoever for any errors, omissions, whether such errors or omissions result from negligence, accident, or any other cause or claims for loss or damages of any kind, including without limitation, indirect or consequential loss or damage arising out of use, inability to use, or about the reliability, -

Tasty Luncheon Catering Menu
TASTY LUNCHEON CATERING MENU PURE VEGETARIAN MENU = 259/Rs PER PLATE. VEG & NON-VEG COMBO = 369/ Rs PER PLATE. LUNCH Or DINNER MENU LUNCH Or DINNER MENU ADD-ON'S COST EXTRA. ADD-ON'S COST EXTRA. 1. Fruit Juice Three Types welcome Drink 1. Fruit Juice Three Types welcome Drink 2. Sweet two types 2. Sweet two types 3. Veg Starter or Bajji 3. Veg Starter or Bajji 4. Veg Dum Biryani or Veg Pulav. 4. Non veg Starter 5. Aloo korma or Paneer curry 5. Non-Veg Dum Biryani or Veg Pulav 6. Non-Veg fry ( Any Of Your Choice ) 6. Poori / Roti / Pulka 7. Non-Veg Curry 7. Veg fry ( Any Of Your Choice ) 8. Poori / Roti / Pulka 8. Veg Curry 9. Aloo kurma Or Paneer Curry 9. Sambar 10. Veg Biryani 10. White Rice 11. Sambar 11. Rotu Pachadi Pickle. 12. White Rice 12. Veg salads. 13. Veg Curry 13. Pappad, Rice Crackers 14. Rotu Pachadi Pickle. 14. Daal 15. Veg salads. 15. Curd. 16. Pappad, Rice Crackers 16. Custurd Fruit salad Ice Cream. 17. Daal 17. Podilu & Ghee 18. Curd. 18. Raita. 19. Custurd Fruit salad Ice Cream. ADD-ONS COST EXTRA 20. Podilu & Ghee ADD-ON'S FLAVOURED RICE= 20/ Rs ADD-ONS VEG CURRY = 40/Rs 21. Raita. ADD-ONS NON-VEG CURRY = 80/Rs ANY ONE FLAVOURED RICE. ADD-ONS COST EXTRA (PULIHORA (LEMMON |TAMARIND | TRADITIONAL ADD-ON'S FLAVOURED RICE= 20/ Rs TEMPLE (DEVUDI PULIHORA). ADD-ON’S VEG CURRY = 40/ Rs SELECT YOUR FAVOURITE VEG CURRY ANY FLAVOURED RICE, TOMATO, MINT, COCONUT OR ADD-ONS ANY PICKLE = 30/Rs CORIANDER) . -

Ambitus Menu Week # 1 Monday Tuesday Wednesday Thursday Friday
Ambitus Menu Week # 1 Monday Tuesday Wednesday Thursday Friday Kichidi with Vegetables Idli With Chutney Poha Idli With Chutney Veg Parata Flavoured Milk Flavoured Milk Flavoured Milk Flavoured Milk Flavoured Milk Green Salad Peanuts Salad Carrot Salad Channa Salad Russian Salad Methi Poori Sesmi Rice Veg Dum Biryani Pav Bhaji Veg Sweet Corn Soup Rajma Masala Plain Rice Chicken Dum Biryani Plain Rice Veg Noodles Plain Rice Veg Curry/Veg Fry Onion Raitha Al00 65 Egg Noodles Louki-Ka-Dal Peppar Rasam Tomato Dal Pappucharu Besibellebath Chutney Chutney Veg Fry Chutney Papad Fryums Fryums Chutney Fryums Curd Rice Curd Rice Fryums Curd Curd Fruit Custord Sweet Curd Sweet Week # 2 Monday Tuesday Wednesday Thursday Friday Oats Pongal With Raitha Idli With Chutney Bread & Jam Idli With Chutney Tomatobath with Chutney Flavoured Milk Flavoured Milk Flavoured Milk Flavoured Milk Flavoured Milk Green Salad Corn Salad Noodles Salad Bhelpoori Onion Salad Pulihora Ajwaine chapathi Veg Biryani Tomato Soup Chicken Butter Masala Plain Rice Chole Masala Egg Biryani Veg Shezwan Noodles Jeera Rice Tendli Coconut Fry Plain Rice Keera Raitha Sambar Rice Plain Rice Veg Masla Curry Masoor Dal Tadka Plain Rice Papad Veg Fry Bangalore Rasam Chutney Veg Fry Curd Rice Drumstick Rasam Fryums Fryums Pappucharu Chutney Curd Fruit Custord Curd Curd Rice Lassi Sweet Sweet Week # 3 Monday Tuesday Wednesday Thursday Friday Uggani Idli With Chutney Semiya Aloo Parata Idli With Chutney Flavoured Milk Flavoured Milk Flavoured Milk Flavoured Milk Flavoured Milk Green Salad -
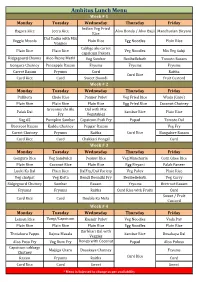
Ambitus Lunch Menu
Ambitus Lunch Menu Week # 1 Monday Tuesday Wednesday Thursday Friday Indian Veg Fried Bagara Rice Jeera Rice Aloo Bonda / Aloo Bajji Manchurian Biryani Rice Dal Tadka with Mix Veggie Masala Plain Rice Egg Noodles Plain Rice Veggies Cabbge alu carrot Plain Rice Plain Rice Veg Noodles Mix Veg Subji capsicum Pakora Ridgegourd Channa Aloo Beans Methi Veg Sambar Besibellebath Tamoto Rasam Curry Dry Gongura Chutney Pineapple Rasam Fryums Fryums Fryums Carrot Rasam Fryums Curd Raitha Curd Rice Curd Rice Curd Sweet Boondi Fruit Custord Week # 2 Monday Tuesday Wednesday Thursday Friday Pulihora Chole Rice Panner Pulov Veg Fried Rice Wada (Gare) Plain Rice Plain Rice Plain Rice Egg Fried Rice Coconut Chutney Greenmirchi Alu Dal with Mix Palak Dal Sambar Rice Plain Rice Fry Vegatables Veg 65 Pumpkin Sambar Capsicum Podi Fry Papad Tamoto Dal Beetroot Rasam Kaddu Chutney Peppar Rasam Veg Fry Carrot Chutney Fryums Raitha Curd Rice Bangalore Rasam Curd Rice Curd Chakkari Pongal Curd Week # 3 Monday Tuesday Wednesday Thursday Friday Gongura Rice Veg Sandwich Peanut Rice Veg Manchuria Corn Ghee Rice Plain Rice Coconut Rice Plain Rice Egg Biryani Palak Paneer Louki Ka Dal Plain Rice DalFry/Dal Variety Veg Pulov Plain Rice Veg chatpat Veg Kofta Bendi Boondhi Fry Besibellebath Veg Curry Ridgegourd Chutney Sambar Rasam Fryums Beetroot Rasam Fryums Fryums Raitha Curd Rice with Fruits Curd Sweet / Fruit Curd Rice Curd Double Ka Meta Custord Week # 4 Monday Tuesday Wednesday Thursday Friday Lemon Rice Vangi/Capsicum Kasmir Pulov Veg Noodles Vada Pav Bath Plain Rice Plain Rice Plain Rice Egg Noodles Plain Rice Darbhari Dal with Thotakura Pappu Rajma Masala Sambar Rice Dosakaya Dal Veggies Aloo Pusa Fry Veg Dum Fry Donda with Coconut Papad Aloo Pulusu Capsicum cabbage Fry Majjiga Charu Dosakaya Chutney Fryums Chutney Rasam Fryums Raitha Curd Rice Curd Curd Rice Curd Sweet Sweet * Menu is Sujected to change as per availability. -

Standardization, Chemical Characterization and Storage Studies of an Instant Pulihora Mix Based on Raw Mango
Indian Journal of Traditional Knowledge Vol.11 (1), January 2012, pp 90-95 Standardization, chemical characterization and storage studies of an instant pulihora mix based on raw mango PG Prabhakara Rao, G Narsing Rao, A Nagender, T Jyothirmayi & A Satyanarayana* Central Food Technological Research Institute-Resource Centre, Council of Scientific and Industrial Research (CSIR), Habshiguda, Uppal Road, Hyderabad 500 007, India E-mail: [email protected] Received 15.10.10; revised Raw mangoes were dehydrated, powdered and mixed with other selected processed spice ingredients to obtain an instant pulihora mix (IPM). The instant mix was found to contain protein and fiber contents of 13.2 and 5.6%, respectively. The mix was a rich source of total polyphenols (636 mg/100 gm) and phosphorous (238 mg/100gm). The mix was found to be a good source of linoleic acid (6.62%). During storage, the free fatty acid and peroxide contents increased from 0.08 to 0.28% and 4.42 to 44.3 meq/kg of fat, respectively. Sorption isotherm revealed that IPM possessed non-hygroscopic nature though it contained 25% of the dehydrated raw mango powder. The critical moisture content of the mix was 12.95% which equilibrated at 65% relative humidity. Sensory analysis of the IPM reconstituted with cooked rice scored ‘good’ (7.7) even after a storage period of six months. Keywords: Mango, Pulihora mix, Physico-chemical analysis, ERH studies, Fatty acid composition, Sensory evaluation IPC Int. Cl.8: B01F, D06M 11/00, C07C 39/00, B65G, A01F 25/00, C01D 3/04, A01G 17/00, A47B 75/00 Raw mango (Mangifera indica L.) is known to possess at household level though such preparations are not good amount of citric and malic acids along with other shelf-stable and are consumed as and when they are nutrients. -

Staples 2 +Jk&Am
Pre-publication version of ‘Civilising tastes: from caste to class in South Indian foodways’ in Food Consumption in Global Perspective: Essays in the Anthropology of Food in Honour of Jack Goody. Editors: Klein JA, Murcott A. 65-86. Palgrave Macmillan, New York 2014. Civilising tastes: from caste to class in South Indian foodways James Staples, Brunel University Abstract Anthropological explorations of food in South Asia are often framed by theories of caste and ritual purity or pollution, with the highest castes characterised as protecting their purity by accepting food from no-one of lower caste status, and those at the bottom accepting food from anyone. The problem with this focus on caste is not that it is misguided per se; many Hindus do indeed regulate their consumption in relation to such concerns, and a quotidian understanding of caste remains vital in understanding how people in India relate to one another. Rather, the problem is that our focus on caste as the defining social institution of India has obscured social relationships defined by other cross-cutting hierarchies that also, and increasingly, reflect and shape Indian foodways. Drawing on prolonged ethnographic fieldwork in Andhra Pradesh, South India, this chapter is concerned with how class in particular – both in terms of economic status and as a marker of distinction – also has profound implications for what people in South India eat, with whom, and why; particularly in the wake of the economic liberalisation that began in the 1990s and the emergence of new foods and tastes ripe for symbolic appropriation. Introduction 1 Pre-publication version of ‘Civilising tastes: from caste to class in South Indian foodways’ in Food Consumption in Global Perspective: Essays in the Anthropology of Food in Honour of Jack Goody. -

Indian Delightful Vegetarian Recipes Chapters
file:///E|/DKS/PUTTAKKA/Puttakka%20Projects/for%20indian%20veg%20recipes%20project/indianvegriceipes.htm INDIAN DELIGHTFUL VEGETARIAN RECIPES CHAPTERS • North Indian Vegetarian Dishes • South Indian Vegetarian Recipes • West Indian Vegetarian Receipes • East Indian Vegetarian Receipes • Andhra Receipe • Bengali Recipes • Goan Recipes • Hyderabadi Recipes • Maharashtrian Recipes • RAJASTHANI RECIPE • Gujarathi Recipes • Punjabhi food • Cabbage Recipes • CAKE RECIPES • CHUTNEY RECIPE file:///E|/DKS/PUTTAKKA/Puttakka%20Projects/for%20indian%20veg%20recipes%20project/indianvegriceipes.htm (1 of 625) [7/4/2012 7:40:38 AM] file:///E|/DKS/PUTTAKKA/Puttakka%20Projects/for%20indian%20veg%20recipes%20project/indianvegriceipes.htm • COOKIE RECIPES • Coconut Recipes • Corn Recipes • DAL RECIPES • PICKLE RECIPE • JAM & JELLY RECIPES • Kebab recipes • PANEER RECIPES (Cottage Cheese) • PUDDING RECIPES • RAITA RECIPES • RICE RECIPES • SALAD RECIPES • SAUCE RECIPE • SOUP RECIPES • DIWALI RECIPES • NAVRATRI RECIPES • ONAM RECIPES file:///E|/DKS/PUTTAKKA/Puttakka%20Projects/for%20indian%20veg%20recipes%20project/indianvegriceipes.htm (2 of 625) [7/4/2012 7:40:38 AM] file:///E|/DKS/PUTTAKKA/Puttakka%20Projects/for%20indian%20veg%20recipes%20project/indianvegriceipes.htm • RAKHI RECIPES • VALENTINES DAY RECIPES ________________________________ I. North Indian Vegetarian Dishes Contents 1. LOBIA RECIPE (Black Eye Beans Curry) 2. Punjabhi Khadhi 3. RAJMA (RED KIDNEY BEANS) RECIPE 4. PESHAWARI CHANA RECIPE 5. RAJASTHANI GATTA CURRY RECIPE 6. METHI KE GATTE RECIPE 7. PAKODI KI KADHI RECIPE 8. RAJASTHANI PAPAD KI SABJI RECIPE 9. CHANE JAISELMER KE RECIPE 10.PANEER KOFTA RECIPE 11. PANEER KORMA RECIPE 12. Paneer Makhani 13.Paneer Pasanda 14. SHAHI PANEER RECIPE 15. PANEER MAKHANA CURRY RECIPE 16. PUNJABI ALOO AMRITSARI RECIPE 17. BHINDI ANARDANA RECIPE 18. PANEER TAASH KABAB RECIPE 19. -

James Monroe Recipe Challenge
James Monroe Recipe Challenge An Owl Cookbook! Eleni M’s Spanakopita Preheat the oven to 350 degrees Fahrenheit. Ingredients First, saute the dill and scallions in 1 tablespoon of olive oil. Slowly add 450 g of uncooked spinach spinach (make sure you have washed and dried your spinach). Add some salt and pepper. 2 scallions Next, strain the mixture to eliminate as much moisture as you can. 80 g dill Then, weigh out 300 g of Feta cheese and crumble up with hands or 300 g Feta cheese fork. Add the Feta cheese to the spinach and dill mixture. Add one egg to the mixture and mix. Add 1/4 cup of panko bread crumbs to the 1 egg mixture and mix in. Set the mixture aside and get your favorite pizza dough or phyllo crust. You are going to need a 9 by 12 inch pan. If 1/4 cup Panko breadcrumbs using dough, split your dough in half, roll out one half of the dough to 1/2 extra-virgin olive oil the thickness that you like. Grease your pan with olive oil. Salt and pepper Then, very carefully place the rolled-out dough in the pan. Add the spinach and Feta mixture on top of the dough. Roll out the other half Sesame seeds of dough and put it on top. Cut off excess dough .Lightly mark squares 1lb dough with a knife so it's easier to cut later on. Sprinkle olive oil and sesame seeds on top. Place the spanakopita in a 350 degree Fahrenheit Flour preheated oven and bake for 40 minutes or until golden brown. -
Newsletter JUNE to NOVEMBER-2018
SANNIHITA GIRLS HOME (STG) Newsletter JUNE TO NOVEMBER-2018 1.Strength of Children We have 110 childrenat presentin our home including newly joined16 children as follow, (From Right K.Ashwini,K. Arthi, L.Renuka,L.Neelima,L.Ashamma, M.Sridevi, N.Keranmai, M.Sangeetha, B.Sandhaya, M.Archana,SK. Sabha fathima,K. Anjali,K. Harika, P.Lathika,P. Anitha,K. Pavani) (b) Educational status:-2018-2019 S.NO CLASS STRENGTH 1 1st 01 2 2nd 03 3 3rd 07 4 4th 16 5 5th 06 6 6th 11 7 7th 10 8 8th 13 9 9th 08 10 10th 16 11 Inter 1st year 07 12 Inter 2nd Year 10 13 Degree 1st Year 01 14 Degree 3rd Year 01 Total 110 Health Status: Growth monitoring with BMI status: We accessed children height and weight last month and found everyone keeping healthy weight. The Menu - Chapathi, Idly, Vada, Poori, Bambino, Wheat Powder, Murmuralu, Beatrice, are fried and cooked to serve. Milk and egg is given as combination in combination. Sometimes items like kichidi, pulihora, Dosa (rice floor), Bonda (roles with dough) are made with rice. Lunch: In a month we serve chicken is served 3 to 4 times, especially on Sunday. On Holidays children are served Veg biryani or any special item from rice. Snack is served after the school hours, around 4.30pm with seasonal fruits Oranges, Guava, Apples and pomegranate is preferred for nutritional values. Children come with surprises for snack and dinner. We want to surprise them with special food like sweets, sprouts, Rajmah and other items like bread and jam.