12 Injection Catheters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CACI Revista 20140228 (2).Indd
ISSN 2250-7531 Colegio Argentino de Cardioangiólogos Intervencionistas CONTIENE ABSTRACTS Revista Argentina de SELECCIONADOS CardioangiologíaSOLACI CACI’14 Intervencionista Enero - Marzo 2014 | Año 5 | Número 1 Artículos Originales Entrenamiento, acreditación y recertifi cación en Cardioan- giología Intervencionista. Veinticinco años de experiencia: 1989-2014 Ruda Vega M, Londero HF, Cherro A Revascularization strategies for patients with multiple ves- sel coronary disease and unprotected left main. A prospec- tive, multicenter and controlled Argentina registry with a co- balt-chromium rapamycin eluting stent, FIREBIRD 2™: Protocol Design and Methods of the ERACI IV Registry Fernández-Pereira C, Santaera O, Larribau M, Haiek C, Sarmiento R, Mie- res J, Lloveras J, Pocoví A, Carlevaro O, Rifourcat I, Chen J, Zheng K, Ro- dríguez-Granillo AM, Antoniucci D, Rodríguez AE; on behalf of ERACI IV Investigators Caso Clínico Bifurcaciones coronarias: técnica stent pull-back modifi cada como estrategia alternativa en pacientes con revascularización previa Dionisio G, Puerta L, Carlevaro O, Kevorkián R, Centeno S Revista Argentina de Cardioangiología Intervencionista Enero - Marzo 2014 | Año 5 | Número 1 Editor en Jefe Marcelo Halac Jorge Leguizamón Eulogio García Traductor Alfredo E. Rodríguez Carlos Miranda Hugo Londero Joan Gómez Alejandro Fernández Pedro Lylyk Editores Asociados Alejandro Peirone Eberhard Grube Representante CACI Esteban Mendaro Liliana Grinfeld Sergio Sierre Luis Guzmán Ernesto M. Torresani Oscar Mendiz Rubén Piraino Pablo Stutzbach Ziyad Hijazi Alejandro Palacios Representante Carrera Antonio Pocoví León Valdivieso Mark Hlatky Juan Parodi Gastón Rodríguez-Granillo Héctor Vetulli Adnan Kastrati UBA-CACI Alfredo E. Rodríguez Gregg Stone José Vicario Kem Morton Guillermo Migliaro Jorge Wisner Omar Santaera Consejo de Redacción Carlos Sztejfman Pedro Lemos Relaciones José Alonso Secretaría Científi ca Alberto Tamashiro Carlos Macaya Institucionales CACI Rosana Ceratto Carla Agatiello David Vetcher Roxana Mehran Lic. -

Trends of Increasing Medical Radiation Exposure in a Population Hospitalized for Cardiovascular Disease (1970–2009)
Trends of Increasing Medical Radiation Exposure in a Population Hospitalized for Cardiovascular Disease (1970–2009) Clara Carpeggiani*, Patrizia Landi, Claudio Michelassi, Paolo Marraccini, Eugenio Picano CNR, Institute of Clinical Physiology, Pisa, Italy Abstract Background: High radiation doses employed in cardiac imaging may increase cancer frequency in exposed patients after decades. The aim of this study was to evaluate the relative trends in medical radiation exposure in a population hospitalized for cardiovascular disease. Methods and Results: An observational single-center study was conducted to examine 16,431 consecutive patients with heart disease admitted to the Italian National Research Council Institute of Clinical Physiology between January 1970 and December 2009. In all patients, the cumulative estimated effective dose was obtained from data mining of electronic records of hospital admissions, adopting the effective dose typical values of the American Heart Association 2009 statement and Mettler’s 2008 catalog of doses. Cumulative estimated effective dose per patient in the last 5 years was 22 (12–42) mSv (median, 25th–75th percentiles), with higher values in ischemic heart disease (IHD), 37 (20–59) vs non-IHD, 13 (8–22) mSv, p,0.001. Trends in radiation exposure showed a steady increase in IHD and a flat trend in non-IHD patients, with variation from 1970–74 to 2005–2009 of +155% for IHD (p,0.001) and 21% in non-IHD (NS). The relative contribution of different imaging techniques was remodeled over time, with nuclear cardiology dominating in 1970s (23% of individual exposure) and invasive fluoroscopy in the last decade (90% of individual exposure). Conclusion: A progressive increase in cumulative estimated effective dose is observed in hospitalized IHD patients. -

Correlation with Left Ventricular Ejection Fraction Determined by Radionudide Ventriculography
J AM cou, CARDIOl 417 1983.1(2):417-20 Reliability of Bedside Evaluation in Determining Left Ventricular Function: Correlation With Left Ventricular Ejection Fraction Determined by Radionudide Ventriculography STEVEN J. MATTLEMAN, MD, A-HAMID HAKKI, MD, ABDULMASSIH S. ISKANDRIAN, MD, FACC, BERNARD L. SEGAL, MD, FACC, SALLY A. KANE, RN Philadelphia. Pennsylvania Ninety-nine patients with chronic coronary artery dis• correctly predicted in only 19 patients (53%) in group ease were prospectively evaluated to determine the reo 2 and in only 9 patients (47%) in group 3. Stepwiselinear liability of historical, physical, electrocardiographic and regression analysis was performed. The single most pre• radiologic data in predicting left ventricular ejection dictive variable was cardiomegaly as seen on chest roent• fraction. The left ventricular ejection fraction measured genography (R2 = 0.52). Four optimal predictive vari• by radionuclide angiography was normal (:::::50%) in 44 ables-cardiomegaly, myocardial infarction as seen on patients (group 1) and abnormal «50%) in 55 patients; electrocardiography, dyspnea and rales-could explain 36 of those 55 patients had an ejection fraction between only 61% of the observed variables in left ventricular 30 and 49% (group 2) and the remaining 19 patients ejection fraction. Thus, radionuclide ventriculography had an ejection fraction of less than 30% (group 3). adds significantly to the discriminant power of the clin• The ejection fraction was correctly predicted in 33 of ical, radiographic and electrocardiographic character• the 44 patients (75%) in group 1 and in 47 of the 55 ization of ventricular function in patients with chronic patients (85%) with abnormal ejection fraction (groups coronary heart disease. -

| Hai Lama Mtandao Wa Wananchi Wana Haiti
|HAI LAMA MTANDAO US009757411B2WA WANANCHI WANA HAITI (12 ) United States Patent ( 10 ) Patent No. : US 9 ,757 ,411 B2 Emanuele et al. ( 45) Date of Patent: Sep. 12, 2017 ( 54 ) POLOXAMER THERAPY FOR HEART 5 ,080 , 894 A 1 / 1992 Hunter .. .. .. .. .. .. .. 424 / 83 5 ,089 , 260 A 2 / 1992 Hunter .. 424 / 83 FAILURE 5 ,523 , 492 A 6 / 1996 Emanuele et al . .. .. .. 568 /606 5 ,567 , 859 A 10 / 1996 Emanuele et al . 568 /624 @(71 ) Applicant : Mast Therapeutics , Inc . , San Diego , 5 ,605 ,687 A 2 / 1997 Lee . 424 /78 . 06 CA (US ) 5 ,691 , 387 A 11/ 1997 Emanuele et al . .. .. .. .. 568 / 723 5 ,696 , 298 A 12 / 1997 Emanuele et al. .. .. 568 /623 @(72 ) Inventors : R . Martin Emanuele , San Diego , CA 5 , 800 , 711 A 9 / 1998 Reeve et al. 210 /639 5 ,990 ,241 A 11/ 1999 Emanuele et al . .. 525 / 88 (US ) ; Santosh Vetticaden , San Diego , RE36 ,665 E 4 /2000 Emanuele et al. 568 /624 CA (US ) ; Patrick Keran , Cardiff , CA RE37 , 285 E 7 / 2001 Emanuele et al. 514 / 723 (US ) 6 , 359, 014 B1 3 / 2002 Emanuele et al. 514 / 723 6 , 747 , 064 B2 6 / 2004 Emanuele et al. 514 / 44 @( 73 ) Assignee : Aires Pharmaceuticals , Inc. , Austin , RE38 , 558 E 7 / 2004 Emanuele et al. .. .. .. .. 568 /623 6 ,761 , 824 B2 7 / 2004 Reeve et al. .. .. .. .. .. .. 210 /639 TX (US ) 6 , 977, 045 B2 12 / 2005 Reeve et al . .. .. .. .. 210 /639 7 , 846 , 426 B2 12 / 2010 Metzger et al. .. .. .. 424 / 78 . 38 ( * ) Notice: Subject to any disclaimer, the term of this 8 ,372 , 387 B2 2 / 2013 Markham et al. -

“Cardiovascular Imaging”
Special Issue on “Cardiovascular Imaging” Aims and Scope Journal of Clinical Trials in Cardiology is a peer reviewed open access journal, exclusively designed for the international Scientific community that addresses ongoing research on Clinical Trials in Cardiology, which aims to provide a unique platform for publishing high quality research work. The journal aims to frame up an outstanding special issue on Cardiovascular Imaging. Cardiovascular Imaging uses the most sophisticated technology offered to capture images of the heart. These different techniques allow physician to make accurate diagnoses and determine the best treatment for each patient. Obtaining and interpreting images of the heart are critical to the successful management of any cardiac disorder. The objective of the special issue is to integrate the growing international community of researchers working on the topics like Coronary Catheterization, Echocardiogram, Intravascular Ultrasound, Positron Emission Tomography and Computed Tomography Angiography. Topics This special issue deals with the topics of the following but not limited to Coronary Catheterization Echocardiogram Intravascular Ultrasound Positron Emission Tomography Computed Tomography Angiography Magnetic Resonance Imaging Chest X-Rays Angiography Intravascular Ultrasonography Endomyocardial Biopsy Spectral Doppler Echocardiography Color Doppler Echocardiography Tissue Doppler Imaging Three-Dimensional Echocardiography Stress Echocardiography Myocardial Perfusion Imaging Cardiac Ventriculography Phonocardiogram -
Lyme Carditis: a Case Involving the Conduction System and Mitral Valve
CASE REPORT Lyme Carditis: A Case Involving the Conduction System and Mitral Valve LAKIR D. PATEL, BS; JAY S. SCHACHNE, MD, FACC ABSTRACT Transthoracic echocardiogram (TTE) revealed preserved left Lyme disease is the most common tick-borne infection ventricular function, severe mitral regurgitation (3+) and a in the Northern hemisphere. Cardiac manifestations of dilated left atrium (Figure 2). Laboratory studies revealed Lyme disease typically include variable atrioventricular anemia (hgb 10.7gm/dL, hematocrit 32.0%), iron defi- nodal block and rarely structural heart pathology. The in- ciency (iron 24µg/dL, saturation 10%) without any history cidence of Lyme carditis may be underestimated based on of melena or hematochezia, and an elevated ESR (80mm/h). current reporting practices of confirmed cases. This case The patient was sent for cardiac catheterization to evaluate of a 59-year-old man with Lyme carditis demonstrates for coronary disease. the unique presentation of widespread conduction sys- Right and left cardiac catheterization demonstrated no tem disease, mitral regurgitation, and suspected isch- flow-limiting, significant, epicardial coronary disease (Fig- emic disease. Through clinical data, electrocardiograms, ure 3). Left ventriculography confirmed moderately severe and cardiac imaging, we show the progression, and reso- mitral regurgitation; right heart pressure recordings showed lution, of a variety of cardiac symptoms attributable to a prominent V wave (25mm Hg) with a normal wedge pres- infection with Lyme. sure. Over the next few days the patient experienced shak- KEYWORDS: Lyme, echocardiography, mitral ing chills and fevers to 101.2 F. Blood cultures in triplicate regurgitation, atrial arrhythmia, conduction and a tick panel were ordered, and the patient was scheduled for transesophageal echocardiogram (TEE) to evaluate for Figure 1. -

Prognostic Relevance of Elevated Pulmonary Arterial Pressure
RESEARCH ARTICLE Prognostic relevance of elevated pulmonary arterial pressure assessed non-invasively: Analysis in a large patient cohort with invasive measurements in near temporal proximity Sebastian Greiner1*, Andreas Jud1, Matthias Aurich1, Christoph Geisenberger2, Lorenz Uhlmann3, Thomas Hilbel1, Meinhard Kieser3, Hugo A. Katus1, Derliz Mereles1 a1111111111 1 Department of Cardiology, Angiology and Pneumology, University of Heidelberg, Heidelberg, Germany, a1111111111 2 Division of Experimental Neurosurgery, University of Heidelberg, Heidelberg, Germany, 3 Institute of Medical Biometry and Informatics, University of Heidelberg, Heidelberg, Germany a1111111111 a1111111111 * [email protected] a1111111111 Abstract OPEN ACCESS Citation: Greiner S, Jud A, Aurich M, Geisenberger Background C, Uhlmann L, Hilbel T, et al. (2018) Prognostic The clinical relevance of non-invasively derived pulmonary arterial pressure (PAP) by relevance of elevated pulmonary arterial pressure assessed non-invasively: Analysis in a large patient Doppler echocardiography (DE) has been questioned in the past. However, transthoracic cohort with invasive measurements in near echocardiography is used as a cornerstone examination for patients with dyspnea and sus- temporal proximity. PLoS ONE 13(1): e0191206. pected pulmonary hypertension (PH). This study aimed to evaluate the prognostic value of https://doi.org/10.1371/journal.pone.0191206 non-invasive assessed PAP in a large population of patients with known or suspected car- Editor: Vincenzo Lionetti, Scuola Superiore diopulmonary disease. Sant'Anna, ITALY Received: September 10, 2017 Methods Accepted: December 30, 2017 The analyses are based on data of patients of a tertiary cardiology center that received right Published: January 19, 2018 heart catheterization (RHC) as well as non-invasively assessed PAP by DE within five days, Copyright: © 2018 Greiner et al. -

The Clinical Course of Takotsubo Syndrome Diagnosed According To
International Journal of Cardiovascular Sciences. 2020; 33(6):637-647 637 ORIGINAL ARTICLE The Clinical Course of Takotsubo Syndrome Diagnosed According to the InterTAK Criteria Nelson Henrique Fantin Fundão,1 Henrique Barbosa Ribeiro,1,2 Carlos de Magalhães Campos,1,3 Vinicius Bocchino Seleme,1 Alexandre de Matos Soeiro,1 Marcelo Luiz Campos Vieira,1,3 Wilson Mathias Jr,1 Ludhmilla Abraão Hajjar,1,2 Expedito E. Ribeiro,1,2 Roberto Kalil Filho1,2 Universidade de São Paulo - Instituto do Coração,1 São Paulo, SP – Brazil. Hospital Sírio-Libanês, São Paulo,2 SP – Brazil. Hospital Israelita Albert Einstein,3 São Paulo, SP – Brazil. Abstract Background: There has been an increase in the number of cases of Takotsubo syndrome (TTS) and of scientific publications on the theme over the last years. However, little is known about the status of this disease in Brazilian hospitals. Objective: To assess mortality and major adverse cardiovascular events (MACE) during hospitalization and follow-up of TTS patients seen in a tertiary hospital in Brazil. Methods: This was a retrospective, observational study on 48 patients. Clinical data, signs and symptoms, complementary tests, MACE and all-cause mortality were assessed on admission and during follow-up. Kaplan-Meier curves were used for analysis of all-cause mortality and risk for MACE at median follow-up. The 95% confidence interval was also calculated for a significance level of 5%. Results: Mean age of patients was 71 years (SD±13 years), and most patients were women (n=41; 85.4%). During hospitalization, four patients (8.3%) died and five (10.4%) developed MACE. -
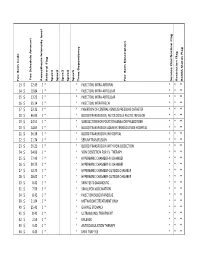
Fee Item C O D E Fee Sch Ed U Le a M O U N T a N Aesth Esia in Ten Sity
Fee Item Code Fee Amount Schedule Fee Anaesthesia Intensity level Referral Flag Spec1 Spec2 Spec3 Spec4 Spec5 Time Dependency Item Description Fee Service Clarification Flag Restriction Flag BCMA Status Flag 13 $ 12.45 0 * * INJECTION, INTRA-ARTERIAL * * ** 14 $ 19.84 0 * * INJECTION, INTRA-ARTICULAR * * ** 15 $ 13.20 0 * * INJECTION, INTRA-ARTICULAR * * ** 16 $ 25.14 0 * * INJECTION, INTRATHECAL * * ** 17 $ 23.32 0 * * INSERTION OF CENTRAL VENOUS PRESSURE CATHETER * * ** 18 $ 46.93 0 * * BLOOD TRANSFUSION, AUTOLOGOUS ASCITIC INFUSION * * ** 19 $ 24.51 0 * * VENESECTION FOR POLYCYTHAEMIA OR PHLEBOTOMY * * ** 20 $ 54.65 0 * * BLOOD TRANSFUSION ADMINISTERED OUTSIDE HOSPITAL * * ** 21 $ 36.38 0 * * BLOOD TRANSFUSION IN HOSPITAL * * ** 22 $ 21.74 0 * * SERUM TRANSFUSION * * ** 23 $ 39.21 0 * * BLOOD TRANSFUSION WITH VEIN DISSECTION * * ** 24 $ 34.96 0 * * VEIN DISSECTION FOR I.V. THERAPY * * ** 25 $ 77.43 7 * * HYPERBARIC CHAMBER-IN CHAMBER * * ** 26 $ 39.75 0 * * HYPERBARIC CHAMBER-IN CHAMBER * * ** 27 $ 52.73 5 * * HYPERBARIC CHAMBER-OUTSIDE CHAMBER * * ** 28 $ 28.00 0 * * HYPERBARIC CHAMBER-OUTSIDE CHAMBER * * ** 30 $ 8.42 0 * * SKIN TESTS-DIAGNOSTIC * * ** 31 $ 7.56 0 * * SMALLPOX-VACCINATION * * ** 34 $ 8.42 0 * * INJECTION SUBCUTANEOUS * * ** 39 $ 21.04 0 * * METHADONE TREATMENT ONLY * * ** 40 $ 25.41 0 * * GAVAGE STOMACH * * ** 41 $ 8.42 0 * * ULTRASOUND, TREATMENT * * ** 42 $ 2.59 0 * * MILEAGE * * ** 43 $ 3.40 0 * * ANTICOAGULATION THERAPY * * ** 44 $ 4.45 0 * * MINI TRAY FEE * * ** 45 $ 15.67 0 * * MANIPULATION THERAPY * * ** 46 -

Quadricuspid Aortic Valve and a Ventricular Septal Defect in a Horse
Michlik et al. BMC Veterinary Research 2014, 10:142 http://www.biomedcentral.com/1746-6148/10/142 CASE REPORT Open Access Quadricuspid aortic valve and a ventricular septal defect in a horse Katarzyna M Michlik1*, Anna K Biazik2, Radomir Z Henklewski2, Marta A Szmigielska2, Józef M Nicpoń1 and Urszula Pasławska1 Abstract Background: Quadricuspid aortic valve (QAV) and ventricular septal defect (VSD) are congenital heart defects and have been described in both human and veterinary medical literature. Case presentation: A 5-year-old half-bred bay stallion was referred for surgical castration. Cardiac murmurs were heard on the presurgical clinical examination and the cardiac examination revealed subcutaneous oedema, tachycardia with a precodrial thrill and a grade 5/6 pansystolic murmur, which was heard on auscultation of the right and left side of the chest. Examination of the B-mode echocardiograms revealed the presence of a QAV (one small cusp, two equal-sized cusps, and one large cusp) and VSD in the membranous portion of the intraventricular septum. These two congenital cardiac defects were accompanied by mild aortic valve regurgitation and severe tricuspid regurgitation. Despite the presence of these cardiac defects, the horse underwent surgical castration under general anesthesia. Surgery, anaesthesia and recovery from anaesthesia were uneventful. The gelding was euthanasied after 17 months because of a progressive loss of body weight, weakness and recumbency. Conclusion: A QAV in combination with VSD in a horse is an interesting finding, because to the best of our knowledge, this has not been previously described in equine literature. Keywords: Horse, Quadricuspid aortic valve, Ventricular septal defect, Congenital heart defect, Echocardiography Background congenital heart disease in horses, among which Arabian A quadricuspid aortic valve (QAV) is a congenital heart horses exhibit particular predisposition to this disorder [9]. -

Characteristics of Coronary Artery Disease Among Patients with Atrial Fibrillation Compared to Patients with Sinus Rhythm
Accepted Manuscript Characteristics of Coronary Artery Disease among Patients with Atrial Fibrillation compared to Patients with Sinus Rhythm Lukas J. Motloch, MD, PhD, Sara Reda, MD, Robert Larbig, MD, Ariane Wolff, MD, Karolina A. Motloch, MD, Bernhard Wernly, MD, Christina Granitz, MD, Michael Lichtenauer, MD, PhD, Martin Wolny, PhD, Uta C. Hoppe, MD PII: S1109-9666(16)30160-9 DOI: 10.1016/j.hjc.2017.03.001 Reference: HJC 145 To appear in: Hellenic Journal of Cardiology Received Date: 22 August 2016 Revised Date: 24 February 2017 Accepted Date: 3 March 2017 Please cite this article as: Motloch LJ, Reda S, Larbig R, Wolff A, Motloch KA, Wernly B, Granitz C, Lichtenauer M, Wolny M, Hoppe UC, Characteristics of Coronary Artery Disease among Patients with Atrial Fibrillation compared to Patients with Sinus Rhythm, Hellenic Journal of Cardiology (2017), doi: 10.1016/j.hjc.2017.03.001. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 ACCEPTED MANUSCRIPT Characteristics of Coronary Artery Disease among Patients with Atrial Fibrillation compared to Patients with Sinus Rhythm Lukas J. Motloch, MD, PhD 1; Sara Reda, MD 1; Robert Larbig, MD 1, 2 ; Ariane Wolff, MD 1; Karolina A. -

Diagnosis of Cardiac Abnormalities in Muscular Dystrophies
medicina Review Diagnosis of Cardiac Abnormalities in Muscular Dystrophies 1,2 3, 3,4 1,5 Elisabeta Bădilă , Iulia Ioana Lungu *, Alexandru Mihai Grumezescu and Alexandru Scafa Udris, te 1 “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (E.B.); [email protected] (A.S.U.) 2 Internal Medicine Department, Clinical Emergency Hospital Bucharest, 014461 Bucharest, Romania 3 Faculty of Applied Chemistry and Materials Science, University Politehnica of Bucharest, 011061 Bucharest, Romania; [email protected] 4 Research Institute of the University of Bucharest—ICUB, University of Bucharest, 050657 Bucharest, Romania 5 Cardiology Department, Clinical Emergency Hospital Bucharest, 014461 Bucharest, Romania * Correspondence: [email protected]; Tel.: +40-21-402-39-97 Abstract: Muscular disorders are mainly characterized by progressive skeletal muscle weakness. There are several aspects that can be monitored, which are used to differentiate between the types of muscular disorders, ranging from the targeted muscle up to the mutated gene. An aspect that holds critical importance when managing muscular dystrophies is that most of them exhibit cardiac abnormalities. Therefore, cardiac imaging is an essential part of muscular disorder monitoring and management. In the first section of the review, several cardiac abnormalities are introduced; afterward, different muscular dystrophies’ pathogenesis is presented. Not all muscular dystrophies necessarily present cardiac involvement; however, the ones that do are linked with the cardiac abnormalities described in the first section. Moreover, studies from the last 3 years on muscular disorders are presented alongside imaging techniques used to determine cardiac abnormalities. Keywords: neuromuscular disorders; cardiac abnormalities; muscular dystrophies; cardiac imaging Citation: B˘adil˘a,E.; Lungu, I.I.; Grumezescu, A.M.; Scafa Udris, te, A.