Photodissociation of the {Heh $^+ $} Molecular
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2. Molecular Stucture/Basic Spectroscopy the Electromagnetic Spectrum
2. Molecular stucture/Basic spectroscopy The electromagnetic spectrum Spectral region fooatocadr atomic and molecular spectroscopy E. Hecht (2nd Ed.) Optics, Addison-Wesley Publishing Company,1987 Per-Erik Bengtsson Spectral regions Mo lecu lar spec troscopy o ften dea ls w ith ra dia tion in the ultraviolet (UV), visible, and infrared (IR) spectltral reg ions. • The visible region is from 400 nm – 700 nm • The ultraviolet region is below 400 nm • The infrared region is above 700 nm. 400 nm 500 nm 600 nm 700 nm Spectroscopy: That part of science which uses emission and/or absorption of radiation to deduce atomic/molecular properties Per-Erik Bengtsson Some basics about spectroscopy E = Energy difference = c /c h = Planck's constant, 6.63 10-34 Js ergy nn = Frequency E hn = h/hc /l E = h = hc / c = Velocity of light, 3.0 108 m/s = Wavelength 0 Often the wave number, , is used to express energy. The unit is cm-1. = E / hc = 1/ Example The energy difference between two states in the OH-molecule is 35714 cm-1. Which wavelength is needed to excite the molecule? Answer = 1/ =35714 cm -1 = 1/ = 280 nm. Other ways of expressing this energy: E = hc/ = 656.5 10-19 J E / h = c/ = 9.7 1014 Hz Per-Erik Bengtsson Species in combustion Combustion involves a large number of species Atoms oxygen (O), hydrogen (H), etc. formed by dissociation at high temperatures Diatomic molecules nitrogen (N2), oxygen (O2) carbon monoxide (CO), hydrogen (H2) nitr icoxide (NO), hy droxy l (OH), CH, e tc. -

First-Principles Calculations of Helium Cluster Formation in Palladium Tritides
FIRST-PRINCIPLES CALCULATIONS OF HELIUM CLUSTER FORMATION IN PALLADIUM TRITIDES A Thesis Presented to The Academic Faculty by Pei Lin In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Physics Georgia Institute of Technology August 2010 FIRST-PRINCIPLES CALCULATIONS OF HELIUM CLUSTER FORMATION IN PALLADIUM TRITIDES Approved by: Professor Mei-Yin Chou, Advisor Professor Andrew Zangwill School of Physics School of Physics Georgia Institute of Technology Georgia Institute of Technology Professor James Gole Professor David Sholl School of Physics School of Chemical and Biomolecular Georgia Institute of Technology Engineering Georgia Institute of Technology Professor Phillip N. First Date Approved: May 10, 2010 School of Physics Georgia Institute of Technology To my beloved family iii ACKNOWLEDGEMENTS I owe my sincere gratitude to all who have helped me, encouraged me, and believed in me through this challenging journey. Foremost, I am deeply indebted to my thesis advisor, Professor Mei-Yin Chou, for every advice and opportunity she provided me through the years of my education. Her exceptional insight and broad-minded guidance led me the way to be a critical-thinking and self-stimulating physicist, which would be my greatest asset in the future. I owe my sincere thanks to Professor Andrew Zangwill, Professor James Gole, Professor Phillip First, and Professor David Sholl for their precious time being in my thesis defense committee. I am thankful to our previous and current group members Dr. Amra Peles, Dr. Li Yang, Dr. Zhu Ma, Dr. Wolfgang Geist, Dr. Alexis Nduwinmana, Dr. Jia-An Yan, Dr. Wen-Ying Ruan, Dr. -

Ionization Dynamics of Helium Dimers in Fast Collisions with Heþþ
week ending PRL 106, 033201 (2011) PHYSICAL REVIEW LETTERS 21 JANUARY 2011 Ionization Dynamics of Helium Dimers in Fast Collisions with Heþþ J. Titze,1 M. S. Scho¨ffler,2 H.-K. Kim,1 F. Trinter,1 M. Waitz,1 J. Voigtsberger,1 N. Neumann,1 B. Ulrich,1 K. Kreidi,3 R. Wallauer,1 M. Odenweller,1 T. Havermeier,1 S. Scho¨ssler,1 M. Meckel,1 L. Foucar,4 T. Jahnke,1 A. Czasch,1 L. Ph. H. Schmidt,1 O. Jagutzki,1 R. E. Grisenti,1 H. Schmidt-Bo¨cking,1 H. J. Lu¨dde,5 and R. Do¨rner1,* 1Institut fu¨r Kernphysik, Goethe-Universita¨t Frankfurt am Main, Max-von-Laue-Str.1, 60438 Frankfurt, Germany 2Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, California, 94720, USA 3GSI Helmholtzzentrum fu¨r Schwerionenforschung GmbH, Planckstr. 1, 64291 Darmstadt, Germany 4Max-Planck-Institut fu¨r Kernphysik, Saupfercheckweg 1, 69117 Heidelberg, Germany 5Institut fu¨r Theoretische Physik, Goethe-Universita¨t Frankfurt am Main, Max-von-Laue-Str. 1, 60438 Frankfurt, Germany (Received 23 September 2010; published 20 January 2011) By employing the cold target recoil ion momentum spectroscopy technique, we have investigated the þ þ (He , He ) breakup of a helium dimer (He2) caused by transfer ionization and double capture in collisions with alpha particles (E ¼ 150 keV=u). Surprisingly, the results show a two-step process as well as a one-step process followed by electron exchange. In addition, interatomic Coulombic decay [L. S. Cederbaum, J. Zobeley, and F. Tarantelli, Phys. Rev. Lett. 79, 4778 (1997).] is observed in an ion collision for the first time. -

Ijmp.Jor.Br V
INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. 10, n. 8, Special Edition Seng 2019 ISSN: 2236-269X DOI: 10.14807/ijmp.v10i8.1046 A NEW HYPOTHESIS ABOUT THE NUCLEAR HYDROGEN STRUCTURE Relly Victoria Virgil Petrescu IFToMM, Romania E-mail: [email protected] Raffaella Aversa University of Naples, Italy E-mail: [email protected] Antonio Apicella University of Naples, Italy E-mail: [email protected] Taher M. Abu-Lebdeh North Carolina A and T State Univesity, United States E-mail: [email protected] Florian Ion Tiberiu Petrescu IFToMM, Romania E-mail: [email protected] Submission: 5/3/2019 Accept: 5/20/2019 ABSTRACT In other papers already presented on the structure and dimensions of elemental hydrogen, the elementary particle dynamics was taken into account in order to be able to determine the size of the hydrogen. This new work, one comes back with a new dynamic hypothesis designed to fundamentally change again the dynamic particle size due to the impulse influence of the particle. Until now it has been assumed that the impulse of an elementary particle is equal to the mass of the particle multiplied by its velocity, but in reality, the impulse definition is different, which is derived from the translational kinetic energy in a rapport of its velocity. This produces an additional condensation of matter in its elemental form. Keywords: Particle structure; Impulse; Condensed matter. [http://creativecommons.org/licenses/by/3.0/us/] Licensed under a Creative Commons Attribution 3.0 United States License 1749 INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. -
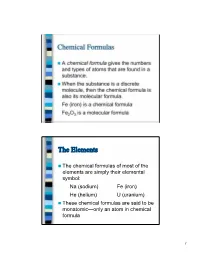
Chemical Formulas the Elements
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula. Fe (iron) is a chemical formula Fe2O3 is a molecular formula The Elements The chemical formulas of most of the elements are simply their elemental symbol: Na (sodium) Fe (iron) He (helium) U (uranium) These chemical formulas are said to be monatomic—only an atom in chemical formula 1 The Elements There are seven elements that occur naturally as diatomic molecules—molecules that contain two atoms: H2 (hydrogen) N2 (nitrogen) O2 (oxygen) F2 (fluorine) Cl2 (chlorine) Br2 (bromine) I2 (iodine) The last four elements in this list are in the same family of the Periodic Table Binary Compounds A binary compound is one composed of only two different types of atoms. Rules for binary compound formulas 1. Element to left in Periodic Table comes first except for hydrogen: KCl PCl3 Al2S3 Fe3O4 2 Binary Compounds 2. Hydrogen comes last unless other element is from group 16 or 17: LiH, NH3, B2H4, CH4 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 Other Compounds For compounds with three or more elements that are not ionic, if it contains carbon, this comes first followed by hydrogen. Other elements are then listed in alphabetical order: C2H6O C4H9BrO CH3Cl C8H10N4O2 3 Other Compounds However, the preceding rule is often ignored when writing organic formulas (molecules containing carbon, hydrogen, and maybe other elements) in order to give a better idea of how the atoms are connected: C2H6O is the molecular formula for ethanol, but nobody ever writes it this way—instead the formula is written C2H5OH to indicate one H atom is connected to the O atom. -

Chem 1A, Fall 2015, Final Exam December 14, 2015 (Prof
Chem 1A, Fall 2015, Final Exam December 14, 2015 (Prof. Head-Gordon)2 Name: ________________________________________ Student ID:_____________________________________ TA: _______________________________ Contents: 14 pages 1. Multiple choice (15 points) 2. Molecular orbitals and quantum mechanics (12 points) 3. Acid-Base Equilibria (13 points) 4. Electrochemistry (12 points) 5. Thermochemistry (12 points) 6. Chemical Kinetics (8 points) Total Points: 72 points (+ 2 bonus points) Instructions: Closed book exam. Basic scientific calculators are OK. Set brains in high gear and go! Thanks for taking our class, and best wishes for success today, and beyond. Some possibly useful facts. Molar volume at STP = 22.4 L 23 -1 N0 = 6.02214 × 10 mol 1 V = 1 J / C -18 -1 -9 Ry = 2.179874 × 10 J = 1312 kJ mol 1 nm = 10 m 15 Ry = 3.28984 × 10 Hz 1 kJ = 1000 J -23 -1 k = 1.38066 × 10 J K 1 atm = 760 mm Hg = 760 torr ≈ 1 bar -34 h = 6.62608 × 10 J s 1 L atm ≈ 100 J T (K) = T (C) + 273.15 -31 me = 9.101939 × 10 kg F = 96,485 C / mol 8 -1 c = 2.99792 × 10 m s -14 Kw = 1.0 × 10 (at 25C) Gas Constant: R = 8.31451 J K-1 mol-1 or -19 1eV = 1.602×10 J R = 8.20578 × 10-2 L atm K-1 mol-1 1 J = 1 kg m2 s-2 1 Some possibly relevant equations: Quantum: Thermodynamics: E = hν λν = c λ deBroglie = h/p = h/mv Ekin (e-) = hν - Φ = hν - hν0 p = mv Particle in a box (1-D Quantum): ΔG° = ΔH° - TΔS° 2 2 2 En = h n /8mL ; n = 1, 2, 3.. -

Experimental Observation of Helium Dimer
Grand Valley State University ScholarWorks@GVSU Peer Reviewed Articles Chemistry Department 12-11-1992 The Weakest Bond: Experimental Observation of Helium Dimer Fei Luo George C. McBane Grand Valley State University, [email protected] Geunsik Kim Clayton F. Giese W. Ronald Gentry Follow this and additional works at: https://scholarworks.gvsu.edu/chm_articles Part of the Biological and Chemical Physics Commons ScholarWorks Citation Luo, Fei; McBane, George C.; Kim, Geunsik; Giese, Clayton F.; and Gentry, W. Ronald, "The Weakest Bond: Experimental Observation of Helium Dimer" (1992). Peer Reviewed Articles. 6. https://scholarworks.gvsu.edu/chm_articles/6 This Article is brought to you for free and open access by the Chemistry Department at ScholarWorks@GVSU. It has been accepted for inclusion in Peer Reviewed Articles by an authorized administrator of ScholarWorks@GVSU. For more information, please contact [email protected]. The weakest bond: Experimental observation of helium dimer Fei Luo, George C. McBane, Geunsik Kim, Clayton F. Giese, and W. Ronald Gentry Chemical Dynamics Laboratory, University of Minnesota, 207 Pleasant Street SE, Minneapolis, Minnesota 55455 (Received 9 November 1992; accepted 11 December 1992) Helium dimer ion was observed after electron impact ionization of a supersonic expansion of helium with translational temperature near 1 mK. The dependence of the ion signal on source pressure, distance from the source, and electron kinetic energy was measured. The signal was determined to arise from ionization of neutral helium dimer. INTRODUCTION servation2 several years ago that it is possible to attain temperatures as low as 0.3 mK in extreme pulsed expan He2 has been the only rare gas dimer not experimen sions of pure helium from room temperature sources. -

Syllabus of Biochemistry (Hons.)
Syllabus of Biochemistry (Hons.) for SEM-I & SEM-II under CBCS (to be effective from Academic Year: 2017-18) The University of Burdwan Burdwan, West Bengal 1 1. Introduction The syllabus for Biochemistry at undergraduate level using the Choice Based Credit system has been framed in compliance with model syllabus given by UGC. The main objective of framing this new syllabus is to give the students a holistic understanding of the subject giving substantial weight age to both the core content and techniques used in Biochemistry. The ultimate goal of the syllabus is that the students at the end are able to secure a job. Keeping in mind and in tune with the changing nature of the subject, adequate emphasis has been given on new techniques of mapping and understanding of the subject. The syllabus has also been framed in such a way that the basic skills of subject are taught to the students, and everyone might not need to go for higher studies and the scope of securing a job after graduation will increase. It is essential that Biochemistry students select their general electives courses from Chemistry, Physics, Mathematics and/or any branch of Life Sciences disciplines. While the syllabus is in compliance with UGC model curriculum, it is necessary that Biochemistry students should learn “Basic Microbiology” as one of the core courses rather than as elective while. Course on “Concept of Genetics” has been moved to electives. Also, it is recommended that two elective courses namely Nutritional Biochemistry and Advanced Biochemistry may be made compulsory. 2 Type of Courses Number of Courses Course type Description B. -

GANPAT UNIVERSITY FACULTY of SCIENCE TEACHING and EXAMINATION SCHEME Programme Bachelor of Science Branch/Spec
GANPAT UNIVERSITY FACULTY OF SCIENCE TEACHING AND EXAMINATION SCHEME Programme Bachelor of Science Branch/Spec. Microbiology Semester I Effective from Academic Year 2015- Effective for the batch Admitted in June 2015 16 Teaching scheme Examination scheme (Marks) Subject Subject Name Credit Hours (per week) Theory Practical Code Lecture(DT) Practical(Lab.) Lecture(DT) Practical(Lab.) CE SEE Total CE SEE Total L TU Total P TW Total L TU Total P TW Total UMBA101FOM FUNDAMENTALS 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - OF MICROBIOLOGY UCHA101GCH GENERAL 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - CHEMISTRY-I UPHA101GPH GENERAL 04 - 04 - - - 04 - 04 - - - 40 60 100 - - - PHYSICS-I UENA101ENG ENGLISH-I 02 - 02 - - - 02 - 02 - - - 40 60 100 - - - OPEN SUBJECT – 1 02 - 02 - - - 02 - 02 - - - 40 60 100 - - - UPMA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I UPCA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I UPPA101PRA PRACTICAL - - - 02 - 02 - - - 04 - 04 - - - - 50 50 MODULE-I Total 16 - 16 06 - 06 16 - 16 12 - 12 200 300 500 - 150 150 GANPAT UNIVERSITY FACULTY OF SCIENCE Programme Bachelor of Science Branch/Spec. Microbiology Semester I Version 1.0.1.0 Effective from Academic Year 2015-16 Effective for the batch Admitted in July 2015 Subject code UMBA 101 Subject Name FUNDAMENTALS OF MICROBIOLOGY FOM Teaching scheme Examination scheme (Marks) (Per week) Lecture(DT) Practical(Lab.) Total CE SEE Total L TU P TW Credit 04 -- -- -- 04 Theory 40 60 100 Hours 04 -- -- -- 04 Practical -- -- -- Pre-requisites: Students should have basic knowledge of Microorganisms and microscopy of 10+2 level. Learning Outcome: The course will help the student to understand basic fundamentals of Microbiology and history of Microbiology. -
Cold Few-Atom Systems with Large S-Wave Scattering Length
Cold Few-Atom Systems With Large s-Wave Scattering Length Doerte Blume, Qingze Guan Experiment: The University of Oklahoma Maksim Kunitski, Reinhard Doerner,… Supported by the NSF. Frankfurt University Cold Few-Atom Systems With Large s-Wave Scattering Length Or Possibly Better: Low-Energy Few-Body Physics Doerte Blume, Qingze Guan Experiment: The University of Oklahoma Maksim Kunitski, Reinhard Doerner,… Supported by the NSF. Frankfurt University Three Few-Body Systems Cold molecular system: Helium dimer, trimer, tetramer,… Nuclear systems: Deuteron, triton, Alpha particle,… Small ultracold alkalis: For fermions, in presence of trap. For bosons, in free space. Pushing toward: Beyond energetics… Beyond statics… Common Characteristic? Large s-Wave Scattering Length 1) Helium-helium (van der Waals length !"#$ ≈ &. ()*): 3 4 1) He- He: )+ = −./. (&)* 4 4 2) He- He: )+ = (0*. 12)* “Naturally” large scattering length. No deep-lying bound states. Tunability? 2) Deuteron (nuclear force around 1 Fermi = (*3(&m): 47* 1) Singlet (4 = *, 6 = (): )+ = −8.. 0Fermi 47( 2) Triplet (4 = (, 6 = *): )+ = &. .1Fermi 3) Ultracold atoms: Feshbach resonances provide enormous tunability. 4Helium-4Helium Example: One Vibrational Bound State Radial wave true He-He potential (calculations to be function: presented use the full potential) F(/% ZR model: (%D->) ;HI(−?/->) 4 4 • He- He bound state energy 89:7;5 = −1.3mK. • No < > * bound states. • Two-body s-wave scattering length -> = 171-*. • Two-body effective range ?;@@ = 15.2-* (alternatively, two- body van der Waals length ?A9B = 5.1-*). 1K = 8.6 × 10−5 eV 1eV= $ × %. '() × (*('+, 1-* = *. .%/01234567 Braaten, Hammer, Physics Reports Three Bosons with 428, 259 (2006). Large |"#|: Efimov Effect ( ( &'( &'(,, $ = + + ∑./0 1(2 3.0 + 1,2 3' − 3( 2 3( − 3, . -
Spectroscopy and Quantum Mechanics of the Helium Dimer
ienc Sc es al J c o i u m r e n a h l C Heidari A, Chem Sci J 2016, 7:2 Chemical Sciences Journal DOI: 10.4172/2150-3494.1000e112 ISSN: 2150-3494 EditorialResearch Article OpenOpen Access Access + Spectroscopy and Quantum Mechanics of the Helium Dimer (He2 ), Neon + + + Dimer (Ne2 ), Argon Dimer (Ar2 ), Krypton Dimer (Kr2 ), Xenon Dimer + + + (Xe2 ), Radon Dimer (Rn2 ) and Ununoctium Dimer (Uuo2 ) Molecular Cations A Heidari* Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA Some of the simplest of all molecules are the Helium dimer, Neon decades. In addition, these cations for the sake of their small atoms and dimer, Argon dimer, Krypton dimer, Xenon dimer, Radon dimer and need to identify and separations gains increasing importance. In this + + + + + + Ununoctium dimer molecular cations, He2 , Ne2 , Ar2 , Kr2 , Xe2 , Rn2 editorial, the molecular structure and thermodynamic parameters and + and Uuo2 . Because of their simplicity and importance, these molecular properties of these cations have also been investigated and calculated cations have received considerable attention from experimentalists (Figures 2 and 3). as well as theoreticians [1-11]. The exact solution of the electronic Schrödinger equation for these cations plays an important role in investigating the molecular structure of more complex systems. In this editorial, some of approximation as well as exact solutions of the Schrödinger equation for these cations have been studied and one the exact solutions have been considered in details. By making use of the perturbation theory, cosmological perturbation theory and also homotopy perturbation method with the help of the linear extrapolation techniques, we have presented a simpler novel method to obtain the numerical exact solution. -

Chemistry in One Dimension Pierre-Fran¸Coisloos,1, A) Caleb J
Chemistry in One Dimension Pierre-Fran¸coisLoos,1, a) Caleb J. Ball,1 and Peter M. W. Gill1, b) Research School of Chemistry, Australian National University, Canberra ACT 0200, Australia We report benchmark results for one-dimensional (1D) atomic and molecular sys- tems interacting via the Coulomb operator jxj−1. Using various wavefunction-type approaches, such as Hartree-Fock theory, second- and third-order Møller-Plesset per- turbation theory and explicitly correlated calculations, we study the ground state of atoms with up to ten electrons as well as small diatomic and triatomic molecules containing up to two electrons. A detailed analysis of the 1D helium-like ions is given and the expression of the high-density correlation energy is reported. We report the total energies, ionization energies, electron affinities and other interesting properties of the many-electron 1D atoms and, based on these results, we construct the 1D analog of Mendeleev's periodic table. We find that the 1D periodic table contains only two groups: the alkali metals and the noble gases. We also calculate the dissociation + 2+ 3+ curves of various 1D diatomics and study the chemical bond in H2 , HeH , He2 , + 2+ H2, HeH and He2 . We find that, unlike their 3D counterparts, 1D molecules are + primarily bound by one-electron bonds. Finally, we study the chemistry of H3 and we discuss the stability of the 1D polymer resulting from an infinite chain of hydrogen atoms. PACS numbers: 31.15.A-, 31.15.V-, 31.15.xt Keywords: one-dimensional atom; one-dimensional molecule; one-dimensional polymer; helium-like ions arXiv:1406.1343v1 [physics.chem-ph] 5 Jun 2014 a)Electronic mail: Corresponding author: [email protected] b)Electronic mail: [email protected] 1 I.