Tribute to Biman Bagchi Laser Spectroscopic Groups of One of Us (G.R.F.), Paul Barbara, and Others
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prospectus 2020
RAMAKRISHNA MISSION VIVEKANANDA CENTENARY COLLEGE Rahara, Kolkata 700 118 An Autonomous College under WBSU College with Potential for Excellence (CPE) Accredited by NAAC with Grade-A With Post-Graduate Section Secured All India Rank 8 in NIRF 2019 Prospectus 2020 History: • The Ramakrishna Mission Boys‟ Home at Rahara, a branch centre of the Ramakrishna Mission, was founded in 1944 as an orphanage with a nucleus of 37 boys rendered orphan by the Great Bengal Famine of 1942-1943. Since then the Home grew in dimensions and activities adhering to the principle of service to mankind in the spirit of worship. Today, the Home is an educational complex with several schools and colleges wherein nearly four thousand students are receiving education and training in different subjects and trades according to the aptitude of each individual. • This College which forms an integral part and unit of this educational complex is owned and managed by the Ramakrishna Mission. The foundation stone of the College was laid by Swami Vireswaranandaji Maharaj, the then General Secretary of the Ramakrishna Mission, on 3rd December, 1961 and the College started functional in July, 1963. • The College was established with a view to commemorating the First Birth Centenary of Swami Vivekananda, the Illustration Patriot-Saint of India and with a view to imparting a general education on a religious background in the light of the teachings of Ramakrishna - Vivekananda so that the young pupils may get ample opportunities to build up their character to make themselves useful to their families and to fulfil at the same time their basic obligation to the country. -
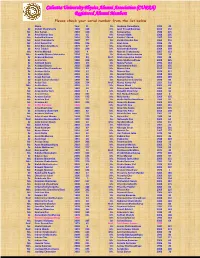
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please Check Your Serial Number from the List Below Name Year Sl
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please check your serial number from the list below Name Year Sl. Dr. Joydeep Chowdhury 1993 45 Dr. Abhijit Chakraborty 1990 128 Mr. Jyoti Prasad Banerjee 2010 152 Mr. Abir Sarkar 2010 150 Dr. Kalpana Das 1988 215 Dr. Amal Kumar Das 1991 15 Mr. Kartick Malik 2008 205 Ms. Ambalika Biswas 2010 176 Prof. Kartik C Ghosh 1987 109 Mr. Amit Chakraborty 2007 77 Dr. Kartik Chandra Das 1960 210 Mr. Amit Kumar Pal 2006 136 Dr. Keya Bose 1986 25 Mr. Amit Roy Chowdhury 1979 47 Ms. Keya Chanda 2006 148 Dr. Amit Tribedi 2002 228 Mr. Krishnendu Nandy 2009 209 Ms. Amrita Mandal 2005 4 Mr. Mainak Chakraborty 2007 153 Mrs. Anamika Manna Majumder 2004 95 Dr. Maitree Bhattacharyya 1983 16 Dr. Anasuya Barman 2000 84 Prof. Maitreyee Saha Sarkar 1982 48 Dr. Anima Sen 1968 212 Ms. Mala Mukhopadhyay 2008 225 Dr. Animesh Kuley 2003 29 Dr. Malay Purkait 1992 144 Dr. Anindya Biswas 2002 188 Mr. Manabendra Kuiri 2010 155 Ms. Anindya Roy Chowdhury 2003 63 Mr. Manas Saha 2010 160 Dr. Anirban Guha 2000 57 Dr. Manasi Das 1974 117 Dr. Anirban Saha 2003 51 Dr. Manik Pradhan 1998 129 Dr. Anjan Barman 1990 66 Ms. Manjari Gupta 2006 189 Dr. Anjan Kumar Chandra 1999 98 Dr. Manjusha Sinha (Bera) 1970 89 Dr. Ankan Das 2000 224 Prof. Manoj Kumar Pal 1951 218 Mrs. Ankita Bose 2003 52 Mr. Manoj Marik 2005 81 Dr. Ansuman Lahiri 1982 39 Dr. Manorama Chatterjee 1982 44 Mr. Anup Kumar Bera 2004 3 Mr. -

Year 2016-17
110 108.97 96 90 70 60.91 DST 50 WB Govt. 30.23 30 20.93 24.39 Project 10 2.96 4.01 4.13 -10 Grant - 2014-15 Grant - 2015-16 Grant - 2016-17 Budget in 2016-17 : DST – 108.97 crores; WB Government – 4.13 crores Web of Science Citation Report (On 19th July, 2017) Result found 1983-2017 No. of Publications : 9939 H Index : 115 Sum of the times cited : 158271 Average citations per item : 15.92 Average citations per year : 4522.03 Performance during the year (2016-17) Publication : 444 Average Impact Factor : 4.4 Ph.D. Degree Awarded : 58 Patent Awarded : 04 Patent Filed : 14 I A C S ANNUAL REPORT 2016 - 2017 INDIAN ASSOCIATION FOR THE CULTIVATION OF SCIENCE Contents From the Director’s Desk ....................................................................... 004 The Past Glory ....................................................................................... 006 The Laurels - Faculty Members ............................................................. 012 The Laurels - Research Fellows ............................................................. 013 Key Committees .................................................................................... 014 Executive Summary ............................................................................... 017 Biological Chemistry .............................................................................. 022 Centre For Advance Materials ............................................................... 031 Director’s Research Unit ....................................................................... -

1. Disposition and Pharmacokinetics
1. DISPOSITION AND PHARMACOKINETICS The disposition and pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds have been investigated in several species and under various exposure conditions. Several reviews on this subject focus on TCDD and related halogenated aromatic hydrocarbons (Neal et al., 1982; Gasiewicz et al., 1983a; Olson et al., 1983; Birnbaum, 1985; van den Berg et al., 1994). The relative biological and toxicological potency of TCDD and related compounds depends not only on the affinity of these compounds for the aryl hydrocarbon receptor (AhR), but on the species-, strain-, and congener-specific pharmacokinetics of these compounds (Neal et al., 1982; Gasiewicz et al., 1983a; Olson et al., 1983; Birnbaum, 1985; van den Berg et al., 1994, DeVito and Birnbaum, 1995). 2,3,7,8-TCDD and other similar compounds discussed here are rapidly absorbed into the body and slowly eliminated, making body burden (bioaccumulation) a reliable indicator of time- integrated exposure and absorbed dose. Because of the slow elimination kinetics, it will be shown in this section that lipid or blood concentrations, which are often measured, are in dynamic equilibrium with other tissue compartments in the body, making the overall body burden and tissue disposition relatively easy to estimate. Finally, it will be shown that body burdens can be correlated with adverse health effects (Hardell et al., 1995; Leonards et al., 1995), further leading to the choice of body burden as the optimal indicator of absorbed dose and potential effects. 1.1. ABSORPTION/BIOAVAILABILITY FOLLOWING EXPOSURE Gastrointestinal, dermal, and transpulmonary absorptions represent potential routes for human exposure to this class of persistent environmental contaminants. -

Annual Report 2017-2018
ANNUAL REPORT IISc 2017-18 INDIAN INSTITUTE OF SCIENCE VISITOR The President of India PRESIDENT OF THE COURT N Chandrasekaran CHAIRMAN OF THE COUNCIL P Rama Rao DIRECTOR Anurag Kumar DEANS SCIENCE: Biman Bagchi ENGINEERING: K Kesava Rao UG PROGRAMME: Anjali A Karande REGISTRAR V Rajarajan Pg 3 IISc RANKED INDIA’S TOP UNIVERSITY In 2016, IISc was ranked Number 1 among universities by the National Institutional Ranking Framework (NIRF) under the auspices of the Ministry of Human Resource Development. It was the first time the NIRF came out with rankings for Indian universities and institutions of higher education. In both 2017 and 2018, the Institute was again ranked first among universities, as well as first in the overall category. CONTENTS Foreword IISc at a Glance 8 1. The Institute 18 Court 5 Council 20 Finance Committee 21 Senate 21 Faculties 21 2. Staff (administration) 22 3. Divisions 25 3.1 Biological Sciences 26 3.2 Chemical Sciences 58 3.3 Electrical, Electronics, and Computer Sciences 86 3.4 Interdisciplinary Research 110 3.5 Mechanical Sciences 140 3.6 Physical and Mathematical Science 180 3.7 Centres under the Director 206 4. Undergraduate Programme 252 5. Awards/Distinctions 254 6. Students 266 6.1 Admissions & On Roll 267 6.2 SC/ST Students 267 6.3 Scholarships/Fellowships 267 6.4 Assistance Programme 267 6.5 Students Council 267 6.6 Hostels 267 6.7 Institute Medals 268 6.8 Awards & Distinctions 269 6.9 Placement 279 6.10 External Registration Program 279 6.11 Research Conferments 280 7. Events 300 7.1 Institute Lectures 310 7.2 Conferences/Seminars/Symposia/Workshops 302 8. -

Unit IV Outiline
CHEMISTRY 111 LECTURE EXAM IV Material PART 1 CHEMICAL EQUILIBRIUM Chapter 14 I Dynamic Equilibrium I. In a closed system a liquid obtains a dynamic equilibrium with its vapor state Dynamic equilibrium: rate of evaporation = rate of condensation II. In a closed system a solid obtains a dynamic equilibrium with its dissolved state Dynamic equilibrium: rate of dissolving = rate of crystallization II Chemical Equilibrium I. EQUILIBRIUM A. BACKGROUND Consider the following reversible reaction: a A + b B ⇌ c C + d D 1. The forward reaction (⇀) and reverse (↽) reactions are occurring simultaneously. 2. The rate for the forward reaction is equal to the rate of the reverse reaction and a dynamic equilibrium is achieved. 3. The ratio of the concentrations of the products to reactants is constant. B. THE EQUILIBRIUM CONSTANT - Types of K's Solutions Kc Gases Kc & Kp Acids Ka Bases Kb Solubility Ksp Ionization of water Kw Hydrolysis Kh Complex ions βη Page 1 General Keq Page 2 C. EQUILIBRIUM CONSTANT For the reaction, aA + bB ⇌ cC + dD The equilibrium constant ,K, has the form: [C]c [D]d Kc = [A]a [B]b D. WRITING K’s 1. N2(g) + 3 H2(g) ⇌ 2 NH3(g) 2. 2 NH3(g) ⇌ N2(g) + 3 H2(g) E. MEANING OF K 1. If K > 1, equilibrium favors the products 2. If K < 1, equilibrium favors the reactants 3. If K = 1, neither is favored F. ACHIEVEMENT OF EQUILIBRIUM Chemical equilibrium is established when the rates of the forward and reverse reactions are equal. CO(g) + 3 H2(g) ⇌ CH4 + H2O(g) Initial amounts moles H 2 Equilibrium amounts moles CO moles CH = moles water 4 Time Page 3 G. -

Statistical Mechanics in Theoretical Chemistry in India
Proc Indian Natn Sci Acad 86 No. 2 June 2020 pp. 903-909 Printed in India. DOI: 10.16943/ptinsa/2019/49727 Review Article Statistical Mechanics in Theoretical Chemistry in India: Past, Present and A Bit of Future AMALENDU CHANDRA1,* and BIMAN BAGCHI2,* 1Department of Chemistry, Indian Institute of Technology Kanpur, Kanpur 208 016, India 2Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore 560 012, India (Received on 03 March 2019; Revised on 25 May 2019; Accepted on 05 June 2019) While the subject of statistical mechanics is intensely active in physics departments across India, with considerable activity even in mathematics departments, the same cannot be said for average chemistry departments in India where statistical mechanics is relatively less taught and even less pursued. This is to be contrasted with USA and many other developed countries (Germany, Israel) where statistical mechanics and spectroscopy are major disciplines in the physical chemistry departments. The situation, however, has changed rapidly in the past few decades. In this article we discuss the developments in this important area in the recent past, with emphasis both on analytical approach and those based on computer simulations. We also attempt to look into future problems where our efforts could be directed fruitfully. Keywords: Theoretical Chemistry; Physical Chemistry; Statistical Mechanics; Molecular Simulations; Chemical Dynamics It is often said that chemistry means chemical of Zeolites and/or other such species by hydrothermal reactions, and most of the chemical reactions occur reactions, one can control the formation of the desired in the solution phase. Many of the biochemical product by tuning the temperature and the processes that sustain all forms of life occur in liquids concentration. -

Elektrochemia Simr 02 En
Electrochemistry course Electrolyte - reminder ACME Faculty, EHVE course Liquid or solid that conducts electricity B.Sc. Studies, II year, IV semester by means of its ions. Ions can move when they have freedom Leszek Niedzicki, PhD, DSc, Eng. of movement. That freedom can be provided by molten salt (ionic liquid) , specific structure of solid enabling ionic mobility or (most commonly) solvation of ions in the solution by solvent Fundamentals of ionics molecules (and as a result - shielding them from counter-ions and causing dissociation). 2 Solvation once more Dynamic equilibrium Disturbance of solvent structure by an ion: • It is a phenomenon observed when on a large scale (e.g. billions of billions of molecules) a statistical equilibrium A – I solvation layer (directly coordinated by a cation) is observed, i.e. mean value of a given parameter is B – II and further solvation layers (attracted steady, but individual molecules often change their electrostatically by a cation and can interact with other solvent state. molecules – e.g. through the hydrogen bonds) • In practice dynamic equilibrium is defined C – solvent structure disturbed by the cation as an equilibrium of two opposite processes, which presence in the vicinity occur at the same rate (in a given conditions). In case D – original solvent structure of solvation solvent molecules are all the time C+ joining and leaving solvation layer (e.g. are knocked A B out of it). However, mean solvent molecules C in solvation layer of a given ion stays the same. D 3 4 Dynamic equilibrium Solvent • In dissociation or solvation case dynamic • Solvent in the electrolyte formation process is equilibrium forms because solvent molecules required to solvate ions (shields them against and ions are bumping on each other association or crystal formation) and dissociate compound into ions (strength of interaction with part (and at the vessel walls) all the time (due to chaotic of the compound tears it from the other part moves, vibrations, etc. -

Raoult's Law – Partition Law
BAE 820 Physical Principles of Environmental Systems Henry’s Law - Raoult's Law – Partition law Dr. Zifei Liu Biological and Agricultural Engineering Henry's law • At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. Pi = KHCi • Where Pi is the partial pressure of the gaseous solute above the solution, C is the i William Henry concentration of the dissolved gas and KH (1774-1836) is Henry’s constant with the dimensions of pressure divided by concentration. KH is different for each solute-solvent pair. Biological and Agricultural Engineering 2 Henry's law For a gas mixture, Henry's law helps to predict the amount of each gas which will go into solution. When a gas is in contact with the surface of a liquid, the amount of the gas which will go into solution is proportional to the partial pressure of that gas. An equivalent way of stating the law is that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. the solubility of gases generally decreases with increasing temperature. A simple rationale for Henry's law is that if the partial pressure of a gas is twice as high, then on the average twice as many molecules will hit the liquid surface in a given time interval, Biological and Agricultural Engineering 3 Air-water equilibrium Dissolution Pg or Cg Air (atm, Pa, mol/L, ppm, …) At equilibrium, Pg KH = Caq Water Caq (mol/L, mole ratio, ppm, …) Volatilization Biological and Agricultural Engineering 4 Various units of the Henry’s constant (gases in water at 25ºC) Form of K =P/C K =C /P K =P/x K =C /C equation H, pc aq H, cp aq H, px H, cc aq gas Units L∙atm/mol mol/(L∙atm) atm dimensionless -3 4 -2 O2 769 1.3×10 4.26×10 3.18×10 -4 4 -2 N2 1639 6.1×10 9.08×10 1.49×10 -2 3 CO2 29 3.4×10 1.63×10 0.832 Since all KH may be referred to as Henry's law constants, we must be quite careful to check the units, and note which version of the equation is being used. -

Measurement of Henry's Law Constant Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DSpace@IZTECH Institutional Repository MEASUREMENT OF HENRY’S LAW CONSTANT OF ORGANOCHLORINATED PESTICIDES A Thesis Submitted to the Graduate School of Engineering and Sciences of zmir Institute of Technology in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE in Chemical Engineering by Serdar Özer July 2005 ZMR We approve the thesis of Serdar ÖZER Date of Signature ................................................................ 26 June 2005 Assist. Prof. Dr. Aysun SOFUOLU Supervisor Department of Chemical Engineering zmir Institute of Technology ................................................................. 26 June 2005 Assoc. Prof. Dr. Funda TIHMINLIOLU Department of Chemical Engineering zmir Institute of Technology ............................................................... 26 June 2005 Assoc. Prof. Dr. Mustafa ODABAI Department of Environmental Engineering 9 September University ................................................................. 26 June 2005 Prof. Dr. Devrim BALKÖSE Head of Department zmir Institute of Technology .............................................................. Assoc. Prof. Dr. Semahat ÖZDEMR Head of the Graduate School ACKNOWLEDGEMENTS I would like to express my gratitude to my advisor, Assist. Prof. Aysun Sofuolu, for her guidance, patience and tolerance. I am grateful to Assoc. Prof. Mustafa Odabaı for his valuable suggestions and guidance. I also would like to give my thanks to Assoc. Prof. Funda Tıhmınlıolu for being in my jury and reccomendations for the corrections. I would like to acknowledge deeply to Banu Çetin, Sevde Bozacıolu, Can Aksakal, and Özge Malay for their encouragement and supports during my study. I would like to appreciate my very special thanks to my girlfriend Baak for her love. ABSTRACT Most of the semi-volatile organic compounds, which are environmentally important, are subject to long range atmospheric transport due to their chemical and physical properties. -

Chemistry 130 Acid and Base Equilibria
Chemistry 130 Acid and Base equilibria Dr. John F. C. Turner 409 Buehler Hall [email protected] Chemistry 130 Acids and bases The Brùnsted-Lowry definition of an acid states that any material that produces the hydronium ion is an acid. A Brùnsted-Lowry acid is a proton donor: + - HA H2 Ol H3 Oaq Aaq + + The hydronium ion, H 3 O a q or H a q has the same structure as ammonia. It is pyramidal and has one lone pair on O. Chemistry 130 Acids and bases The Brùnsted-Lowry definition of a base states that any material that accepts a proton is a base. A Brùnsted-Lowry base is a proton acceptor: - - + Baq H2 Ol HOaq BHaq In aqueous solution, a base forms the hydroxide ion: Chemistry 130 Conjugate acids and bases For any acid-base reaction, the original acid and base are complemented by the conjugate acid and conjugate base: - + NH3aq H2Ol HOaq NH4aq On the RHS, On the LHS, Water is the proton donor Hydroxide ion is the proton acceptor Ammonia is the proton acceptor Ammonium ion is the proton donor Water and hydroxide ion are conjugate acid and base Ammonia and ammonium ion are also conjugate acid and base Chemistry 130 Conjugate acids and bases The anion of every acid is the conjugate base of that acid The cation of every base is the conjugate acid of that base Acid-base conjugates exist due to the dynamic equilibrium that exists in solution. - + NH3aq H2Ol HOaq NH4aq - + NH3aq H2Ol HOaq NH4aq Chemistry 130 Acid and base constants We use equilibrium constants to define the position of equilibrium and to reflect the dynamic nature of the system. -

CHM 1046 FINAL REVIEW Prepared & Presented By: Marian Ayoub
CHM 1046 FINAL REVIEW Prepared & Presented By: Marian Ayoub PART II Chapter Description 14 Chemical Equilibrium 15 Acids and Bases 16 Acid-Base Equilibrium 17 Solubility and Complex-Ion Equilibrium 19 Electrochemistry 20 Nuclear Chemistry CH. 14 CHEMICAL EQUILIBRIUM • DYNAMIC EQUILIBRIUM: WHEN FORWARD AND REVERSE REACTION RATES ARE THE SAME. *PURE SOLIDS AND LIQUIDS DO NOT APPEAR IN THE EQUILIBRIUM CONSTANT EXPRESSION (ANY K OR Q) • THE EQUILIBRIUM CONSTANT KC: THE VALUE OBTAINED FOR THE EQUILIBRIUM-CONSTANT EXPRESSION WHEN EQUILIBRIUM CONCENTRATIONS ARE SUBSTITUTED. • THE EQUILIBRIUM CONSTANT KP: THE EQUILIBRIUM CONSTANT EXPRESSION FOR A GASEOUS REACTION IN TERMS OF PARTIAL PRESSURES. ΔN FOR GASES: KP = KC (RT) , WHERE ΔN = DIFFERENCE OF COEFFICIENT. Ch. 14 Chemical Equilibrium CALCULATING VALUES OF K • IF 2 OR MORE REACTIONS ARE ADDED TO ACHIEVE A GIVEN REACTION, THE EQUILIBRIUM CONSTANT FOR THE GIVEN EQUATION EQUALS THE PRODUCT OF THE EQUILIBRIUM CONSTANTS (K) OF THE ADDED EQUATIONS. K1xK2 • IF REACTION IS REVERSED YOU TAKE THE INVERSE OF ORIGINAL K OF REACTION. 1/K • IF MULTIPLIED OR DIVIDED, RAISE K TO THAT POWER. EX: MULTIPLY BY 2 K2 EX: DIVIDE BY 3 K1/3 Ch. 14 Chemical Equilibrium USING QC TO DETERMINE THE DIRECTION OF EQUILIBRIUM • REACTION QUOTIENT (QC): THE INITIAL REACTION RATE OF A REACTION. ITS NOT A CONSTANT BUT DYNAMIC. • THE RELATIONSHIP BETWEEN QC AND KC GIVES THE DIRECTION OF THE EQUILIBRIUM. • IF KC > QC EQUILIBRIUM IS IN THE FORWARD DIRECTION. • IF KC = QC REACTION IS IN EQUILIBRIUM. • IF KC < QC EQUILIBRIUM IS IN THE REVERSE DIRECTION. • IF QC = 0 ONLY REACTANTS ARE PRESENT. • IF QC = ∞ ONLY PRODUCTS ARE PRESENT.