Abstract Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The SDA Church in Southern Asia Division Depends Heavily Upon Its Members for the Return of Tithes
1 P. H. Lail General Manager Northern India Union Headquarters of SDA, New Delhi. Oriental Watchman Publishing House ,Pune. Spicer Memorial College, Pune. Northeast India Union H Neville 0. Matthews W.G. Jenson President 1990-94. President Central India Union Headquarters of SDA, Pune S.G. Mahapure President R.D. Riches E.B. Matthews President 1990-92 Adventist Communication Centre, Pune. Manager M.E.Cherian President L.C. Cooper James M. Campbell Secretary, 1990-94. Secretary D. Kujur esident Nepal Bhutan Johnson Koilpillai I. Nagabhushana Rao Treasurer, 1990-93. Treasurer Southern Asia Division Administrative Complex of SDA, HOS121.. Darters of SDA, Shillong. J.M. Dkhar President hn Willmott esident, 1990-93 W.G. Kore South India Union Headquarters of SDA, Bangalore. President THE SEVENTH-DAY ADVENTIST CHURCH IN SOUTHERN ASIA The Challenging Years 1990-95 IMAGES II THE SEVENTH-DAY ADVENTIST CHURCH IN SOUTHERN ASIA Ji wants the Church to 6e in the future and how we are tofulfi /the mission for which it has been called info existence. One Aundredyeczrs is not an insigml2cant period even in the life Van insfithtion such as the Church andg fit hadheen a period fgrowth and development it goday the Church in (Southern Msia must Aaoe been ofnecessity a periodofmalurinyfor the look tats uponA e /cis/ one hundred years fits existence, Church. c5o as we enter the second century of our of rowth and develop men!, of god's providences, of..7fi's existence, a very pertinent vita/ question arises and care am/protection, as evell as 6/essings with a deep sense assumes great significance. -

Edelgive Hurun India Philanthropy List 2020 10 November 2020
EDELGIVE HURUN INDIA PHILANTHROPY LIST 2020 10 NOVEMBER 2020 Press Release Report EdelGive Hurun India Philanthropy List 2020 Key Highlights WITH A DONATION OF INR 7,904 CRORE, AZIM PREMJI,75, WAS ‘INDIA’S MOST GENEROUS’. HE DONATED INR 22 CRORES PER DAY! HCL’S SHIV NADAR, 75, WAS SECOND WITH INR 795 CRORE DONATION WITH A DONATION OF INR 27 CRORE, AMIT CHANDRA,52, AND ARCHANA CHANDRA, 49, OF A.T.E. CHANDRA FOUNDATION ARE THE FIRST AND ONLY PROFESSIONAL MANAGERS TO EVER ENTER THE EDELGIVE HURUN INDIA PHILANTHROPY LIST. INDIAN PHILANTHROPY STATS AT A RECORD HIGH; NO. OF INDIVIDUALS WHO HAVE DONATED MORE THAN INR 10 CRORE INCREASED BY 100% OVER THE LAST 2 YEARS, FROM 37 TO 78 THIS YEAR LED BY SD SHIBULAL, 65, WHO DONATED INR 32 CRORES, 28 NEW ADDITIONS TO THE LIST; TOTAL DONATIONS BY NEW ADDITIONS AT INR 313 CRORE WITH A DONATION OF INR 458 CRORE, INDIA’S RICHEST MAN, MUKESH AMBANI,63, CAME THIRD WITH A DONATION OF INR 276 CRORE, KUMAR MANGALAM BIRLA, 53, OF ADITYA BIRLA GROUP DEBUTS THE TOP 5 AND IS THE YOUNGEST IN TOP 10 YOUNGEST: BINNY BANSAL, 37, DEBUTED THE EDELGIVE HURUN INDIA PHILANTHROPY LIST WITH A DONATION OF INR 5.3 CRORES WITH 90 PHILANTHROPISTS CUMULATIVELY DONATING INR 9,324 CRORES, EDUCATION THE MOST FAVOURED CAUSE. WITH 84 DONORS, HEALTHCARE REGISTERED A 111% INCREASE IN CUMULATIVE DONATION, FOLLOWED BY DISASTER RELIEF & MANAGEMENT, WHICH HAD 41 DONORS, REGISTERING A CUMULATIVE DONATION OF INR 354 CRORES OR AN INCREASE OF 240% 3 OF INFOSYS’S CO-FOUNDERS MADE THE LIST, WITH NANDAN NILEKANI, S GOPALAKRISHNAN AND SD SHIBULAL, -

Curriculum Vitae
CURRICULUM VITAE Dr. G.SHEELA Assistant Professor (PG), Department of Studies in Education University of Mysore Manasagangotri Mysore Address : # 212, I „G‟ Cross, III Stage, IV Block, Basaveshwaranagar, Bangalore- 560079. Phone No : 080 23482466 Mobile : 9448241293 Date of Birth : 6-5-1972 Category : General Merit Educational Qualifications : M.Sc., M.Ed., PGDHE., Ph.D NET –UGC & SLET- Govt. of Karnataka: Qualified Ph.D. in Education : “A Study of Effectiveness of Synectics Model of Teaching Science on Creativity and Problem Solving Ability of Secondary School Students.” Computer Skills : Operating System : MS-DOS, WINDOWS Programming Language : D base, C language Packages : MS-Word, Excel, Power Point 1 Teaching Experience: Institution Post Held Class Subjects dealt Period Universtiy of Lecturer M.Ed Teacher Education 24-3-2006 Mysore Educational Psycology till date Curriculum & Evaluation Special Education Basaveshwara Principal B.Ed Ednl. Psychology and 24_ 8-2005 to College of Statistics 24-3-2006 Education, Bangalore Bangalore Guest Lecturer M.Ed Advanced Methods of 23-11-2001 to University Teaching 30 –6-2006 Research Methodology and Statistics Measurement and Evaluation Curriculum Development Karnataka State (Bangalore M.Ed Research Methodology & 2001 onwards Open University, University Study I year Statistics Mysore, Centre) Lecturer Educational Technology 2001 onwards M.Ed Multimedia in Teaching II year Sharada Vilas Lecturer B.Ed Methods of teaching 1 academic year Teacher‟s Mathematics College, Mysore Educational Psychology Action Research Sacred Hearts Lecturer TCH Content cum 1 year 10 months Teacher Training Methodology of Institute teaching Physics Research Experience : Institution Post Held Project Period N.C.E.R.T (R.I.E.), Junior Project Fellow SOPT 6 months Mysore N.C.E.R.T (R.I.E.), Junior Project Fellow E.R.I.C 1 Year Mysore N.C.E.R.T (R.I.E.), Junior Project Fellow E.R.I.C 4 months Mysore 2 Book Publications: “Synectics Model of Teaching”, Published by Anmol Publications Pvt. -

Annual Report 2012-13.Pdf
CONTENTS Board of Directors 2 Management’s Discussion and Analysis 3 Directors’ Report 19 Corporate Governance 31 CEO Declaration on Code of Conduct 52 CEO & CFO Certificate 52 Financial Statements 53 Indian GAAP Standalone 58 Consolidated Statements 103 Statement under Section 212 148 Statement regarding Subsidiary Companies 150 1 Content.p65 1 10/7/2013, 3:16 PM BOARD OF DIRECTORS MR. SHIV NADAR Chairman & Chief Strategy Officer Ms. ROSHNI NADAR MALHOTRA Non-Executive Director MR. VINEET NAYAR Non-Executive Director MR. AMAL GANGULI Non-Executive & Independent Director MR. KEKI MISTRY Non-Executive & Independent Director MR. R. SRINIVASAN Non-Executive & Independent Director AuditorsAuditorsAuditors Ms. ROBIN ABRAMS M/s. S. R. Batliboi & Co. LLP Non-Executive & Independent Director Chartered Accountants Gurgaon MR. SOSALE SHANKARA SASTRY Non-Executive & Independent Director BankersBankersBankers Citibank, N.A. MR. SRIKANT MADHAV DATAR Global Corporate & Investment Banking Non-Executive & Independent Director DLF Centre, 5th Floor Parliament Street MR. SUBRAMANIAN MADHAVAN New Delhi-110001 Non-Executive & Independent Director Deutsche Bank AG MR. SUBROTO BHATTACHARYA Corp. Office - DLF Square Non-Executive & Independent Director 4th floor, Jacaranda Marg, DLF City, Phase - II MR. SUDHINDAR KRISHAN KHANNA Gurgaon-122002 Non-Executive & Independent Director Standard Chartered Bank Corporate & Institutional Banking Credit Operations, India H -2, Connaught Circus New Delhi-110001 State Bank of India Corporate Accounts Group Branch 11th /12th Floor, Jawahar Vyapar Bhawan 1, Tolstoy Marg New Delhi-110001 2 2 HCLTechno MDA 2-18.p65 2 10/7/2013, 3:16 PM MANAGEMENT DISCUSSION AND ANALYSIS Investors are cautioned that this discussion contains forward looking statements that involve risks and uncertainties. -

Prospectus 2020
RAMAKRISHNA MISSION VIVEKANANDA CENTENARY COLLEGE Rahara, Kolkata 700 118 An Autonomous College under WBSU College with Potential for Excellence (CPE) Accredited by NAAC with Grade-A With Post-Graduate Section Secured All India Rank 8 in NIRF 2019 Prospectus 2020 History: • The Ramakrishna Mission Boys‟ Home at Rahara, a branch centre of the Ramakrishna Mission, was founded in 1944 as an orphanage with a nucleus of 37 boys rendered orphan by the Great Bengal Famine of 1942-1943. Since then the Home grew in dimensions and activities adhering to the principle of service to mankind in the spirit of worship. Today, the Home is an educational complex with several schools and colleges wherein nearly four thousand students are receiving education and training in different subjects and trades according to the aptitude of each individual. • This College which forms an integral part and unit of this educational complex is owned and managed by the Ramakrishna Mission. The foundation stone of the College was laid by Swami Vireswaranandaji Maharaj, the then General Secretary of the Ramakrishna Mission, on 3rd December, 1961 and the College started functional in July, 1963. • The College was established with a view to commemorating the First Birth Centenary of Swami Vivekananda, the Illustration Patriot-Saint of India and with a view to imparting a general education on a religious background in the light of the teachings of Ramakrishna - Vivekananda so that the young pupils may get ample opportunities to build up their character to make themselves useful to their families and to fulfil at the same time their basic obligation to the country. -

Team India ISEF 2014 26 Flag Off Ceremony News and Events 26 28 Events Diary
Indo-US Science & Technology Forum Newsletter of IUSSTF Volume 6 (1) | May 2014 HERE COMES THE SUN SOLAR ENERGY RESEARCH INSTITUTE FOR Indo-US Science and Technology Forum INDIA AND THE UNITED STATES CONTENTS cover story SERIIUS 04 Here comes the Sun 12 features Indo-U.S. Joint Center 10 A Class Apart reports 3rd Bangalore Cognition Workshop 14 Good memories, Ignited minds Student–Speak 16 Giving wings to talent! 15 Padma Awards 22 Laurels Galore! 24 Indo-US Energy Dialogue Team India ISEF 2014 26 Flag Off Ceremony news and events 26 28 Events Diary 30 2 Connect • May 2014 From the Editor-in-Chief Editor-in-Chief Science and technology collaboration between India and the US Rajiv Sharma has been a very vibrant and multi-faceted one. The major principle behind collaboration in the 21st century has been that joint programs Executive Director, IUSSTF are jointly selected, expenses co-funded and any outcome co shared. The Indo-US Science and Technology Forum established Editorial Advisory Group in March 2000 through an inter-governmental agreement is a co Samuel Kotis funded and co-governed autonomous organization, which has now Deputy Minister Counselor become a special vehicle to administer several such programs. Environment A shining example of Indo-US S&T collaboration is the Science and Technology Affairs establishment of a “virtual” Joint Clean Energy Research and U.S. Embassy, New Delhi Development Center (JCERDC), to promote joint clean energy research in the areas of solar energy, second generation bio-fuels Michael Cheetham and energy efficiency of buildings. This 5-year 100 Mn US$ Director, India Science and Technology initiative is funded in public- private partnership mode with 25 Mn US$ each by the Indian Government (through Partnership (INSTP) Department of Science and Technology and Department of Biotechnology) and the US Government (through the Department of Energy) with a matching grant from partnering industries. -

Short+Sweet Theatre Festival South India (2016) About Short+Sweet 2016
Short+Sweet Theatre Festival South India (2016) About Short+Sweet 2016 Short+Sweet (originally known as Short & Sweet) is a multi-form arts platform presenting festivals in theatre, dance, music-theatre, comedy and cabaret across Australia and Asia. Short+Sweet's vision statement is: a more creative world ten minutes at a time. The flagship festival is Short+Sweet Theatre Sydney, the largest ten-minute play festival in the world. Short+Sweet Theatre was founded at the Newtown Theatre in Sydney, Australia in January 2002 by Mark Cleary, beginning with the first ever Short+Sweet Theatre festival. Festivals are now held across the globe. Short+Sweet Theatre Sydney is now the largest ten-minute theatre festival in the world, with around 180 new plays being produced every season. There are many unique elements to this festival, some of which are, a strict time-limit, limit of one play per playwright, one play per director and two plays per actor, people's choice voting and the competitive aspect of the festival where plays are chosen from each week to progress to the Gala Finals. Short+Sweet South India started as Short+Sweet Chennai in 2011. Since 2014, the festival has opened to entries from the four southern states in India. The jury members for the weeks are not known to the public, and consist of a good mix of connoisseurs from diverse fields. The festival is presented in association with Blu Lotus Foundation and Alliance Francaise of Madras. Currently, Meera Krishnan, senior programme coordinator at Prakriti Foundation, is the Festival Director. -

HCL-TECH-2009-2010.Pdf
CONTENTS Board of Directors 2 Management’s Discussion and Analysis 3 Directors’ Report 16 Corporate Governance Report 33 Declaration on Code of Conduct 57 CEO and CFO Certif cate 57 Financial Statements 59 Indian GAAP Standalone 64 Consolidated Statements 103 Statement under Section 212 140 Statement regarding Subsidiary Companies 142 BOARD OF DIRECTORS MR. SHIV NADAR Chairman & Chief Strategy off cer MR. VINEET NAYAR CEO & Whole-time Director MR. T. S. R. SUBRAMANIAN Non-Executive Director MS. ROBIN ABRAMS Non-Executive Director MR. AJAI CHOWDHRY Non-Executive Director MR. SUBROTO BHATTACHARYA Non-Executive Director MR. AMAL GANGULI Non-Executive Director MR. P. C. SEN Non-Executive Director Auditors S.R. Batliboi & Co. Chartered Accountants Gurgaon Bankers Citibank, N.A. Global Corporate & Investment Banking DLF Centre, 5th Floor Parliament Street New Delhi–110001 Deutsche Bank AG Corp. Off ce – DLF Square 4th f oor, Jacaranda Marg, DLF City, Phase – II Gurgaon-122002 Standard Chartered Bank Corporate & Institutional Banking Credit Operations, India H -2, Connaught Circus New Delhi–110001 State Bank of India Corporate Accounts Group Branch 11th / 12th Floor Jawahar Vyapar Bhawan 1, Tolstoy Marg New Delhi-110001 2 MANAGEMENT DISCUSSION AND ANALYSIS Investors are cautioned that this discussion contains forward looking statements that involve risks and uncertainties. When words like ‘anticipate’, ‘believe’, ‘estimate’, ‘intend’, ‘will’, and ‘expect’ and other similar expressions are used in this discussion, they relate to the Company or its business and are intended to identify such forward-looking statements. The Company undertakes no obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. -
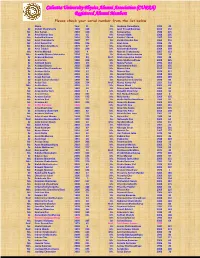
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please Check Your Serial Number from the List Below Name Year Sl
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please check your serial number from the list below Name Year Sl. Dr. Joydeep Chowdhury 1993 45 Dr. Abhijit Chakraborty 1990 128 Mr. Jyoti Prasad Banerjee 2010 152 Mr. Abir Sarkar 2010 150 Dr. Kalpana Das 1988 215 Dr. Amal Kumar Das 1991 15 Mr. Kartick Malik 2008 205 Ms. Ambalika Biswas 2010 176 Prof. Kartik C Ghosh 1987 109 Mr. Amit Chakraborty 2007 77 Dr. Kartik Chandra Das 1960 210 Mr. Amit Kumar Pal 2006 136 Dr. Keya Bose 1986 25 Mr. Amit Roy Chowdhury 1979 47 Ms. Keya Chanda 2006 148 Dr. Amit Tribedi 2002 228 Mr. Krishnendu Nandy 2009 209 Ms. Amrita Mandal 2005 4 Mr. Mainak Chakraborty 2007 153 Mrs. Anamika Manna Majumder 2004 95 Dr. Maitree Bhattacharyya 1983 16 Dr. Anasuya Barman 2000 84 Prof. Maitreyee Saha Sarkar 1982 48 Dr. Anima Sen 1968 212 Ms. Mala Mukhopadhyay 2008 225 Dr. Animesh Kuley 2003 29 Dr. Malay Purkait 1992 144 Dr. Anindya Biswas 2002 188 Mr. Manabendra Kuiri 2010 155 Ms. Anindya Roy Chowdhury 2003 63 Mr. Manas Saha 2010 160 Dr. Anirban Guha 2000 57 Dr. Manasi Das 1974 117 Dr. Anirban Saha 2003 51 Dr. Manik Pradhan 1998 129 Dr. Anjan Barman 1990 66 Ms. Manjari Gupta 2006 189 Dr. Anjan Kumar Chandra 1999 98 Dr. Manjusha Sinha (Bera) 1970 89 Dr. Ankan Das 2000 224 Prof. Manoj Kumar Pal 1951 218 Mrs. Ankita Bose 2003 52 Mr. Manoj Marik 2005 81 Dr. Ansuman Lahiri 1982 39 Dr. Manorama Chatterjee 1982 44 Mr. Anup Kumar Bera 2004 3 Mr. -

Year 2016-17
110 108.97 96 90 70 60.91 DST 50 WB Govt. 30.23 30 20.93 24.39 Project 10 2.96 4.01 4.13 -10 Grant - 2014-15 Grant - 2015-16 Grant - 2016-17 Budget in 2016-17 : DST – 108.97 crores; WB Government – 4.13 crores Web of Science Citation Report (On 19th July, 2017) Result found 1983-2017 No. of Publications : 9939 H Index : 115 Sum of the times cited : 158271 Average citations per item : 15.92 Average citations per year : 4522.03 Performance during the year (2016-17) Publication : 444 Average Impact Factor : 4.4 Ph.D. Degree Awarded : 58 Patent Awarded : 04 Patent Filed : 14 I A C S ANNUAL REPORT 2016 - 2017 INDIAN ASSOCIATION FOR THE CULTIVATION OF SCIENCE Contents From the Director’s Desk ....................................................................... 004 The Past Glory ....................................................................................... 006 The Laurels - Faculty Members ............................................................. 012 The Laurels - Research Fellows ............................................................. 013 Key Committees .................................................................................... 014 Executive Summary ............................................................................... 017 Biological Chemistry .............................................................................. 022 Centre For Advance Materials ............................................................... 031 Director’s Research Unit ....................................................................... -

List of the Various Endowment Awards and the Previous Awardees of the Respective Awards
.List of the various Endowment Awards and the previous Awardees of the respective Awards 1. Acharya P. C. Ray Memorial Award: (Annual) Specialization: This award is to be given to an eminent chemist of any specialization. List of Acharya P. C. Ray Memorial Past Lecturers Year Recipient & Place Year Recipient & Place 1968 Prof. R. N. Chakraborty, Calcutta* 1998 Prof. G. Govil, Mumbai 1969 Prof. S. C. Bhattacharyya, Calcutta 1999 Prof. P. Natarajan, Chennai 1970 Dr. Sukh Dev, Baroda 2000 Prof. D. S. Bhakuni, Lucknow 1971 Prof. D. K. Banerjee, Bangalore 2001 Prof. G. K. Trivedi, Mumbai 1972 Dr. Nitya Nand, Lucknow 2002 Prof. N. G. Kundu, Kolkata 1973 Prof. S. Rangaswami, Delhi 2003 Prof. Rakesh Bohra, Jaipur 1974 Prof.(Mrs.) Asima Chatterjee, Calcutta* 2004 Prof. V. S. Chauhan, New Delhi 1976 Dr. T.R. Govindachari, Madras* 2005 Prof. Sudarsan Arora, Pune 1977 Prof. R. C. Mehrotra, Jaipur* 2006 Prof. U. C. Agarwala, Kanpur 1978 Dr. B. D. Tilak, Poona 2007 Prof. N. K. Kausik, Delhi 1979 Prof. P. K. Bhattacharya, Bangalore* 2008 Prof. Tulsi Mukherjee, Mumbai. 1980 Prof. Arun K. Dey, Allahabad* 2009 Dr. K. N. Ganesh, NCL, Pune 1981 Prof. S. Swaminathan, Madras 2010 Dr. Ashok Misra, Bengaluru 1982 Prof. K. C. Joshi, Jaipur 2011 Prof. R.V. Hosur, Mumbai 1983 Prof. R. C. Kapoor, Jodhpur* 2012 Prof. A.K. Mishra, IIT, Chennai 1984 Prof. C. N. R. Rao, Bangalore 2013 Prof. Deb Shankar Ray, Kolkata 1985 Prof. U. R. Ghatak, Calcutta* 2014 Prof. G.D. Yadav, ICT, Mumbai 1986 Prof. D. Banerjea, Calcutta 2015 Prof. Mihir Kanti Chaudhuri, Tezpur 1987 Prof. -

Dear Aspirant with Regard
DEAR ASPIRANT HERE WE ARE PRESENTING YOU A GENRAL AWERNESS MEGA CAPSULE FOR IBPS PO, SBI ASSOT PO , IBPS ASST AND OTHER FORTHCOMING EXAMS WE HAVE UNDERTAKEN ALL THE POSSIBLE CARE TO MAKE IT ERROR FREE SPECIAL THANKS TO THOSE WHO HAS PUT THEIR TIME TO MAKE THIS HAPPEN A IN ON LIMITED RESOURCE 1. NILOFAR 2. SWETA KHARE 3. ANKITA 4. PALLAVI BONIA 5. AMAR DAS 6. SARATH ANNAMETI 7. MAYANK BANSAL WITH REGARD PANKAJ KUMAR ( Glory At Anycost ) WE WISH YOU A BEST OF LUCK CONTENTS 1 CURRENT RATES 1 2 IMPORTANT DAYS 3 CUPS & TROPHIES 4 4 LIST OF WORLD COUNTRIES & THEIR CAPITAL 5 5 IMPORTANT CURRENCIES 9 6 ABBREVIATIONS IN NEWS 7 LISTS OF NEW UNION COUNCIL OF MINISTERS & PORTFOLIOS 13 8 NEW APPOINTMENTS 13 9 BANK PUNCHLINES 15 10 IMPORTANT POINTS OF UNION BUDGET 2012-14 16 11 BANKING TERMS 19 12 AWARDS 35 13 IMPORTANT BANKING ABBREVIATIONS 42 14 IMPORTANT BANKING TERMINOLOGY 50 15 HIGHLIGHTS OF UNION BUDGET 2014 55 16 FDI LLIMITS 56 17 INDIAS GDP FORCASTS 57 18 INDIAN RANKING IN DIFFERENT INDEXS 57 19 ABOUT : NABARD 58 20 IMPORTANT COMMITTEES IN NEWS 58 21 OSCAR AWARD 2014 59 22 STATES, CAPITAL, GOVERNERS & CHIEF MINISTERS 62 23 IMPORTANT COMMITTEES IN NEWS 62 23 LIST OF IMPORTANT ORGANIZATIONS INDIA & THERE HEAD 65 24 LIST OF INTERNATIONAL ORGANIZATIONS AND HEADS 66 25 FACTS ABOUT CENSUS 2011 66 26 DEFENCE & TECHNOLOGY 67 27 BOOKS & AUTHOURS 69 28 LEADER”S VISITED INIDIA 70 29 OBITUARY 71 30 ORGANISATION AND THERE HEADQUARTERS 72 31 REVOLUTIONS IN AGRICULTURE IN INDIA 72 32 IMPORTANT DAMS IN INDIA 73 33 CLASSICAL DANCES IN INDIA 73 34 NUCLEAR POWER