Statistical Mechanics in Theoretical Chemistry in India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prospectus 2020
RAMAKRISHNA MISSION VIVEKANANDA CENTENARY COLLEGE Rahara, Kolkata 700 118 An Autonomous College under WBSU College with Potential for Excellence (CPE) Accredited by NAAC with Grade-A With Post-Graduate Section Secured All India Rank 8 in NIRF 2019 Prospectus 2020 History: • The Ramakrishna Mission Boys‟ Home at Rahara, a branch centre of the Ramakrishna Mission, was founded in 1944 as an orphanage with a nucleus of 37 boys rendered orphan by the Great Bengal Famine of 1942-1943. Since then the Home grew in dimensions and activities adhering to the principle of service to mankind in the spirit of worship. Today, the Home is an educational complex with several schools and colleges wherein nearly four thousand students are receiving education and training in different subjects and trades according to the aptitude of each individual. • This College which forms an integral part and unit of this educational complex is owned and managed by the Ramakrishna Mission. The foundation stone of the College was laid by Swami Vireswaranandaji Maharaj, the then General Secretary of the Ramakrishna Mission, on 3rd December, 1961 and the College started functional in July, 1963. • The College was established with a view to commemorating the First Birth Centenary of Swami Vivekananda, the Illustration Patriot-Saint of India and with a view to imparting a general education on a religious background in the light of the teachings of Ramakrishna - Vivekananda so that the young pupils may get ample opportunities to build up their character to make themselves useful to their families and to fulfil at the same time their basic obligation to the country. -
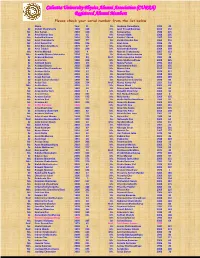
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please Check Your Serial Number from the List Below Name Year Sl
Calcutta University Physics Alumni Association (CUPAA) Registered Alumni Members Please check your serial number from the list below Name Year Sl. Dr. Joydeep Chowdhury 1993 45 Dr. Abhijit Chakraborty 1990 128 Mr. Jyoti Prasad Banerjee 2010 152 Mr. Abir Sarkar 2010 150 Dr. Kalpana Das 1988 215 Dr. Amal Kumar Das 1991 15 Mr. Kartick Malik 2008 205 Ms. Ambalika Biswas 2010 176 Prof. Kartik C Ghosh 1987 109 Mr. Amit Chakraborty 2007 77 Dr. Kartik Chandra Das 1960 210 Mr. Amit Kumar Pal 2006 136 Dr. Keya Bose 1986 25 Mr. Amit Roy Chowdhury 1979 47 Ms. Keya Chanda 2006 148 Dr. Amit Tribedi 2002 228 Mr. Krishnendu Nandy 2009 209 Ms. Amrita Mandal 2005 4 Mr. Mainak Chakraborty 2007 153 Mrs. Anamika Manna Majumder 2004 95 Dr. Maitree Bhattacharyya 1983 16 Dr. Anasuya Barman 2000 84 Prof. Maitreyee Saha Sarkar 1982 48 Dr. Anima Sen 1968 212 Ms. Mala Mukhopadhyay 2008 225 Dr. Animesh Kuley 2003 29 Dr. Malay Purkait 1992 144 Dr. Anindya Biswas 2002 188 Mr. Manabendra Kuiri 2010 155 Ms. Anindya Roy Chowdhury 2003 63 Mr. Manas Saha 2010 160 Dr. Anirban Guha 2000 57 Dr. Manasi Das 1974 117 Dr. Anirban Saha 2003 51 Dr. Manik Pradhan 1998 129 Dr. Anjan Barman 1990 66 Ms. Manjari Gupta 2006 189 Dr. Anjan Kumar Chandra 1999 98 Dr. Manjusha Sinha (Bera) 1970 89 Dr. Ankan Das 2000 224 Prof. Manoj Kumar Pal 1951 218 Mrs. Ankita Bose 2003 52 Mr. Manoj Marik 2005 81 Dr. Ansuman Lahiri 1982 39 Dr. Manorama Chatterjee 1982 44 Mr. Anup Kumar Bera 2004 3 Mr. -

Year 2016-17
110 108.97 96 90 70 60.91 DST 50 WB Govt. 30.23 30 20.93 24.39 Project 10 2.96 4.01 4.13 -10 Grant - 2014-15 Grant - 2015-16 Grant - 2016-17 Budget in 2016-17 : DST – 108.97 crores; WB Government – 4.13 crores Web of Science Citation Report (On 19th July, 2017) Result found 1983-2017 No. of Publications : 9939 H Index : 115 Sum of the times cited : 158271 Average citations per item : 15.92 Average citations per year : 4522.03 Performance during the year (2016-17) Publication : 444 Average Impact Factor : 4.4 Ph.D. Degree Awarded : 58 Patent Awarded : 04 Patent Filed : 14 I A C S ANNUAL REPORT 2016 - 2017 INDIAN ASSOCIATION FOR THE CULTIVATION OF SCIENCE Contents From the Director’s Desk ....................................................................... 004 The Past Glory ....................................................................................... 006 The Laurels - Faculty Members ............................................................. 012 The Laurels - Research Fellows ............................................................. 013 Key Committees .................................................................................... 014 Executive Summary ............................................................................... 017 Biological Chemistry .............................................................................. 022 Centre For Advance Materials ............................................................... 031 Director’s Research Unit ....................................................................... -

Academy News
Proc Indian Natn Sci Acad 85 No. 4 December 2019 pp. 1067-1090 Printed in India. ACADEMY NEWS INSA MEETINGS to Professor Tarun Kant, FNA, Professor Emeritus, Department of Civil Engineering, Several meetings were held during April 08-10, 2019 Indian Institute of Technology Bombay, Powai, in the Academy premises. Mumbai. These included meetings of the different Sectional 4. Professor K Naha Memorial Medal to Committees for recommending names of Young Professor SS Rai, FNA, Professor Emeritus, Scientist Awardees and for the first round of short- Department of Earth and Climate Science, listing of nominations for INSA Fellowship. The Indian Institute of Science Education & Advisory Boards for the various INSA Awards also Research (IISER), Pune. met. These were followed by meetings of the Council and General Body. (C) Endowment Lectures INSA Medal/Lecture Awards 2019 5. Professor Darshan Ranganathan Memorial Lecture to Professor Gaiti Hasan, FNA, The Academy at its General Body Meeting on April National Centre for Biological Sciences, Tata 10, 2019 announced the following six medal/lecture Institute of Fundamental Research, Bengaluru. Awards for 2019. In addition, a new endowment award named International Award Professor TV Desikachary Memorial Medal was also 1. PMS Blackett Memorial Lecture to Sir Tom instituted. The Medal will be awarded to an eminent L Blundell, FNA, Emeritus Professor and scientist for his outstanding contributions in any area Director of Research, Department of of Biological Sciences. The award carries an Biochemistry, University of Cambridge, honorarium of Rs. 25,000/, a bronze medal and a Cambridge. citation. The first medal will be awarded in 2020. -

Annual Report 2017-2018
ANNUAL REPORT IISc 2017-18 INDIAN INSTITUTE OF SCIENCE VISITOR The President of India PRESIDENT OF THE COURT N Chandrasekaran CHAIRMAN OF THE COUNCIL P Rama Rao DIRECTOR Anurag Kumar DEANS SCIENCE: Biman Bagchi ENGINEERING: K Kesava Rao UG PROGRAMME: Anjali A Karande REGISTRAR V Rajarajan Pg 3 IISc RANKED INDIA’S TOP UNIVERSITY In 2016, IISc was ranked Number 1 among universities by the National Institutional Ranking Framework (NIRF) under the auspices of the Ministry of Human Resource Development. It was the first time the NIRF came out with rankings for Indian universities and institutions of higher education. In both 2017 and 2018, the Institute was again ranked first among universities, as well as first in the overall category. CONTENTS Foreword IISc at a Glance 8 1. The Institute 18 Court 5 Council 20 Finance Committee 21 Senate 21 Faculties 21 2. Staff (administration) 22 3. Divisions 25 3.1 Biological Sciences 26 3.2 Chemical Sciences 58 3.3 Electrical, Electronics, and Computer Sciences 86 3.4 Interdisciplinary Research 110 3.5 Mechanical Sciences 140 3.6 Physical and Mathematical Science 180 3.7 Centres under the Director 206 4. Undergraduate Programme 252 5. Awards/Distinctions 254 6. Students 266 6.1 Admissions & On Roll 267 6.2 SC/ST Students 267 6.3 Scholarships/Fellowships 267 6.4 Assistance Programme 267 6.5 Students Council 267 6.6 Hostels 267 6.7 Institute Medals 268 6.8 Awards & Distinctions 269 6.9 Placement 279 6.10 External Registration Program 279 6.11 Research Conferments 280 7. Events 300 7.1 Institute Lectures 310 7.2 Conferences/Seminars/Symposia/Workshops 302 8. -

Tribute to Biman Bagchi Laser Spectroscopic Groups of One of Us (G.R.F.), Paul Barbara, and Others
Special Issue Preface pubs.acs.org/JPCB Tribute to Biman Bagchi laser spectroscopic groups of one of us (G.R.F.), Paul Barbara, and others. The solvation dynamics was reported to have times scales faster than dielectric relaxation, which posed a challenge to theorists for proper explanations. Just before joining IISc, Bagchi, in collaboration with G.R.F. and David Oxtoby, had developed a theory of dipolar solvation using a continuum model of the solvent and a frequency dependent dielectric function. The theory predicted a solvation time that was faster than the dielectric relaxation time of the solvent, thus providing an explanation of the experimentally observed fast relaxation of the time dependent solvation energy. The continuum theory was subsequently generalized by Bagchi and co-workers in many different directions such as incorporation of multi-Debye relaxation, non-Debye relaxation, inhomogeneity of the medium around a solute, etc. Still, being based on continuum models, all these extensions lacked the molecularity of the solvent. Besides, these theories considered only the rotational motion of solvent molecules because the dynamics came through the frequency Photo by S. R. Prasad dependence of the long wavelength dielectric function. Micro- rofessor Biman Bagchi has made pivotal contributions to the scopic theories based on molecular solvent models also started P area of dynamics of chemical and biological systems in an coming from other groups; however, these microscopic theories academic career spanning more than three decades. He has been also included only the rotational motion of solvent molecules. a great teacher and mentor for a large number of young These rotation-only microscopic theories predicted an average theoretical physical chemists of India and a creative and insightful solvation time that was longer than the long-wavelength fi collaborator with leading scientists worldwide. -

Mihir Chowdhury (1937–2017): Scientist and Teacher Extraordinaire
PERSONAL NEWS Mihir Chowdhury (1937–2017): scientist and teacher extraordinaire Scientific and technical biography throwing light on how Mihir Chowdhury’s research interest evolved dur- ing his active years in science and how he innovated in both teaching and research. Henry Smith wrote ‘A teacher can affect (1966–76) of his tenure there. At the teaching even during the busiest part of eternity; he does not know when his in- same time, he was moving to IACS to his career as a scientist. He was no ora- fluence comes to an end’. The sad de- succeed Professor S. R. Palit, the undis- tor; it was the depth of his analysis and mise of Professor Mihir Chowdhury puted emperor of polymer chemistry in crisp logic-based articulation of a subject (formerly Head, Department of Physical India in those days. The Department was from scratch that enthralled the students Chemistry, Indian Association for the indeed well known for its contributions and made the classroom alive. Cultivation of Science (IACS), Kolkata) to polymer and solution chemistry. on 28 March 2017 brought to the fore the Chowdhury’s task was cut out: he would deep truth of Smith’s observation1. Pro- have to reshape, remodel and refashion The scientist tracted and debilitating illness kept him the Department into a modern centre of away from active science for over a dec- physical chemistry. He had the clear The research activities of Chowdhury ade or so; yet the news of his passing vision and meticulous planning that the were varied, full of ingenuity, novelty away evoked a spontaneous and acute task called for. -

Mihir Chowdhury (1937–2017)
PERSONAL NEWS Mihir Chowdhury (1937–2017) Professor Mihir Chowdhury, one of the at Pennsylvania State University (with Presidency College in 1971 (during the pioneers in modern physical chemistry in L. Goodman, 1961–62) and at Chicago turbulent Naxalite period). Many of us India, passed away on 28 March 2017. (with D. S. McClure, 1962–64), USA. still recall his courses on thermodynam- He is widely respected for introducing There he developed a strong interest in ics, quantum chemistry, group theory and lasers and many state-of-the-art tech- high-resolution spectroscopy. NMR spectroscopy. His classes used to niques in physical chemistry research in be long (2 h instead of the scheduled 1 h) India. He along with M. V. George, J. P. and still the students thronged in large Mittal, V. Krishnan, late P. Natarajan numbers. He always encouraged excel- and V. Ramamurthy ushered in modern lence. I remember in a college examina- photochemistry in India. tion, he deducted 10 marks because I Born in Dhaka (Bangladesh) on 15 picked up an easy question. July 1937, he was the youngest child of In 1976, Chowdhury moved back to Jogendra Kumar Chowdhury and Indira. IACS as the Professor and Head of the He had three sisters and one brother. His Department of Physical Chemistry. elder brother, Subir Chowdhury is a pio- Within a decade, he transformed it as one neer in operations research in India and of the leading centres of physical chem- his eldest sister Amita Datta was a pro- istry in India with five fellows of the fessor of economics at the Presidency Indian Academy of Sciences and four College, Kolkata. -

Volume Content 1..11
Journal of Chemical Sciences [Formerly: Proceedings of the Indian Academy of Sciences (Chemical Sciences)] Volume 124, 2012 CONTENTS Special issue on Structure, Reactivity and Dynamics Foreword 11–12 Dynamics of atom tunnelling in a symmetric double well coupled to an asymmetric double well: The case of malonaldehyde S Ghosh and S P Bhattacharyya 13–19 Shape change as entropic phase transition: A study using Jarzynski relation Moupriya Das, Debasish Mondal and Deb Shankar Ray 21–28 Fitness landscapes in natural rocks system evolution: A conceptual DFT treatment Soma Duley, Jean-Louis Vigneresse and Pratim Kumar Chattaraj 29–34 Hydrogen bonded networks in formamide [HCONH2]n (n ¼ 1 − 10) clusters: A computational exploration of preferred aggregation patterns A Subha Mahadevi, Y Indra Neela and G Narahari Sastry 35–42 Use of an intense microwave laser to dissociate a diatomic molecule: Theoretical prediction of dissociation dynamics Amita Wadehra and B M Deb 43–50 + A quantum-classical simulation of a multi-surface multi-mode nuclear dynamics on C6H6 incorporating degeneracy among electronic states Subhankar Sardar and Satrajit Adhikari 51–58 Understanding proton affinity of tyrosine sidechain in hydrophobic confinement T G Abi, T Karmakar and S Taraphder 59–63 Full dimensional quantum scattering study of the H2 þ CN reaction S Bhattacharya, A Kirwai, Aditya N Panda and H-D Meyer 65–73 Dynamics of atomic clusters in intense optical fields of ultrashort duration Deepak Mathur and Firoz A Rajgara 75–81 Bhageerath—Targeting the near impossible: -

Year Book of the Indian National Science Academy
AL SCIEN ON C TI E Y A A N C A N D A E I M D Y N E I A R Year Book B of O The Indian National O Science Academy K 2019 2019 Volume I Angkor, Mob: 9910161199 Angkor, Fellows 2019 i The Year Book 2019 Volume–I S NAL CIEN IO CE T A A C N A N D A E I M D Y N I INDIAN NATIONAL SCIENCE ACADEMY New Delhi ii The Year Book 2019 © INDIAN NATIONAL SCIENCE ACADEMY ISSN 0073-6619 E-mail : esoffi [email protected], [email protected] Fax : +91-11-23231095, 23235648 EPABX : +91-11-23221931-23221950 (20 lines) Website : www.insaindia.res.in; www.insa.nic.in (for INSA Journals online) INSA Fellows App: Downloadable from Google Play store Vice-President (Publications/Informatics) Professor Gadadhar Misra, FNA Production Dr VK Arora Shruti Sethi Published by Professor Gadadhar Misra, Vice-President (Publications/Informatics) on behalf of Indian National Science Academy, Bahadur Shah Zafar Marg, New Delhi 110002 and printed at Angkor Publishers (P) Ltd., B-66, Sector 6, NOIDA-201301; Tel: 0120-4112238 (O); 9910161199, 9871456571 (M) Fellows 2019 iii CONTENTS Volume–I Page INTRODUCTION ....... v OBJECTIVES ....... vi CALENDAR ....... vii COUNCIL ....... ix PAST PRESIDENTS OF THE ACADEMY ....... xi RECENT PAST VICE-PRESIDENTS OF THE ACADEMY ....... xii SECRETARIAT ....... xiv THE FELLOWSHIP Fellows – 2019 ....... 1 Foreign Fellows – 2019 ....... 154 Pravasi Fellows – 2019 ....... 172 Fellows Elected (effective 1.1.2019) ....... 173 Foreign Fellows Elected (effective 1.1.2019) ....... 177 Fellowship – Sectional Committeewise ....... 178 Local Chapters and Conveners ...... -

Abstract Book
26th CRSI-National Symposium in Chemistry CRSI-NSC-26 February 07-09, 2020 Abstract Book Jointly Organized by Department of Chemistry VIT Vellore, CRSI, & RSC, UK Contents Welcome Organizing Committee Message from Founder President, CRSI Message from Founder Chancellor, VIT Message from Pro Vice Chancellor, VIT Message from President, CRSI Message from Dean, School of Advance Science Acknowledgements Program Abstracts President’s Lecture Animesh Chakravorty Endowment Lecture CRSI Medal Lecture C. N. R. Rao National Prize for Chemical Research CRSI Honorary Fellowship Lecture C. N. R. Rao Award Lecture Silver Medal Lecture Mizushima - Raman Lecture Third Charusita Chakravarty Memorial Lecture Lifetime Achievement Award Lecture Bronze Medal Lectures Invited Lectures Abstracts Posters Welcome Message from Organizers On behalf of organizing committee of the 26th National Symposium in Chemistry (NSC) and 14th Royal Society of Chemistry Joint Symposium under the auspices of the Chemical Research Society of India (CRSI), we are delighted to welcome you to the symposium and to the Vellore Institute of Technology Vellore. The 26th CRSI-NSC meeting is attended by a large number of scientists, academicians and students from universities and academic/research institutions spread across the length and breadth of India. The meeting is attended by nearly 200 registered participants. The symposium has 34 lectures that include CRSI medal lectures, special lectures. More than 130 posters will be presented by faculty and students. The Royal Society of Chemistry, UK has been partnering with CRSI in organizing a one day symposium, along with the CRSI National Symposium over the years. This year also, there will be a CRSI-RSC symposium on February 6, 2020, preceding the CRSI National Symposium at Vellore. -

Archive-Mashelkar Endowment Lectures (May 2011-Jan 2015) Sr No
Archive-Mashelkar Endowment Lectures (May 2011-Jan 2015) Sr No. Date Speaker Name Title and Abstract 1 30-May-11 Prof. Biman Bagchi Title: Single Molecular View of Biological Processes : Protein-DNAInteraction, Enzyme Kinetics and Kinetic Proof Reading Abstract : Complexity of biological processes often does not allow a molecular level understanding of important dynamical events. However, recent single molecule spectroscopic studies have revealed new and fascinating aspects of several age-old problems that motivated new theoretical developments. In this talk I shall discuss our recent work on several such important biological processes, namely (a) the mechanism of diffusion of a non-specifically bound protein along DNA in its search for the specific binding site, and (b) the role of enzyme fluctuations in enzyme catalysis and, (c) explicit role of water in enzyme kinetics, and (d) theory of kinetic proof reading or, theory of lack of errors in biological synthesis within cells. 2 16-Jun-11 Prof Yamuna Krishnan Title: Molecular DNA devices in Living Systems Abstract : DNA has attractive physicochemical characteristics such as robust thermal and hydrolytic stability. It also has desirable structural characteristics stemming from predictable and specific recognition properties that give rise to a highly regular helical structure which behaves as a rigid rod on length scales upto ~50 nm. Since these rigid rods may be welded together by complementary base-pairing, DNA is now taking on a new aspect where it is finding use as a construction element to build exquisitely complex architectures on the nanoscale. This field is called structural DNA nanotechnology. We have been interested in architecturally simple, yet functional DNA-based molecular devices.