The Prevalence of Pseudoxanthoma Elasticum-Like Connective Tissue Changes in an Oral Biopsy Service Thesis Presented in Partial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RD-Action Matchmaker – Summary of Disease Expertise Recorded Under
Summary of disease expertise recorded via RD-ACTION Matchmaker under each Thematic Grouping and EURORDIS Members’ Thematic Grouping Thematic Reported expertise of those completing the EURORDIS Member perspectives on Grouping matchmaker under each heading Grouping RD Thematically Rare Bone Achondroplasia/Hypochondroplasia Achondroplasia Amelia skeletal dysplasia’s including Achondroplasia/Growth hormone cleidocranial dysostosis, arthrogryposis deficiency/MPS/Turner Brachydactyly chondrodysplasia punctate Fibrous dysplasia of bone Collagenopathy and oncologic disease such as Fibrodysplasia ossificans progressive Li-Fraumeni syndrome Osteogenesis imperfecta Congenital hand and fore-foot conditions Sterno Costo Clavicular Hyperostosis Disorders of Sex Development Duchenne Muscular Dystrophy Ehlers –Danlos syndrome Fibrodysplasia Ossificans Progressiva Growth disorders Hypoparathyroidism Hypophosphatemic rickets & Nutritional Rickets Hypophosphatasia Jeune’s syndrome Limb reduction defects Madelung disease Metabolic Osteoporosis Multiple Hereditary Exostoses Osteogenesis imperfecta Osteoporosis Paediatric Osteoporosis Paget’s disease Phocomelia Pseudohypoparathyroidism Radial dysplasia Skeletal dysplasia Thanatophoric dwarfism Ulna dysplasia Rare Cancer and Adrenocortical tumours Acute monoblastic leukaemia Tumours Carcinoid tumours Brain tumour Craniopharyngioma Colon cancer, familial nonpolyposis Embryonal tumours of CNS Craniopharyngioma Ependymoma Desmoid disease Epithelial thymic tumours in -
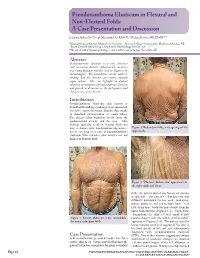
Pseudoxanthoma Elasticum in Flexural and Non-Flexural Folds: a Case Presentation and Discussion
Pseudoxanthoma Elasticum in Flexural and Non-Flexural Folds: A Case Presentation and Discussion Shahrzad Akbary, BS,* Joseph Machuzak, DO, FAOCD,** Richard Bernert, MD, FASDP*** *Medical Student, 4th year, Midwestern University - Arizona College of Osteopathic Medicine, Glendale, AZ **Board Certified Dermatologist, MacKenzie Dermatology, Prescott, AZ ***Board Certified Dermatopathologist, Arizona Dermatopathology, Scottsdale, AZ Abstract Pseudoxanthoma elasticum is a rare, inherited connective-tissue disorder. Characteristic cutaneous and biopsy findings typically lend to diagnosis by dermatologists. The presentation can be subtle or striking, and the disorder can involve multiple organ systems. Here we highlight an atypical cutaneous presentation of pseudoxanthoma elasticum and provide a discussion on the pathogenesis and characteristics of the disorder. PseudoxanthomaIntroduction elasticum, also known as Grönblad-Strandberg syndrome, is an autosomal- recessive connective-tissue disorder that results in abnormal mineralization of elastic fibers. The disease often manifests in the skin, the cardiovascular system, and the eyes. Skin findings typically occur in flexural folds and have a characteristic xanthomatous appearance; Figure 2. Redundant folds; note sparing of the herein, we present a case of pseudoxanthoma upper back. elasticum with extensive skin involvement not limited to flexural folds. Figure 3. “Plucked chicken skin” appearance in the right axilla and chest. folds; the patient denied any history of extreme weight loss. The patient’s redundant skin was diffusely distributed to his neck, underarms, thorax, abdomen, and mid to lower back. Very little of his upper body was spared aside from his upper back and face (Figures 1, 2). Upon closer examination, the skin revealed small yellow Figure 1. Thorax, abdomen, arms; remarkable papules characteristic of a “plucked chicken skin” for many redundant folds. -

Pseudoxanthoma Elasticum
Pseudoxanthoma elasticum Description Pseudoxanthoma elasticum (PXE) is a progressive disorder that is characterized by the accumulation of deposits of calcium and other minerals (mineralization) in elastic fibers. Elastic fibers are a component of connective tissue, which provides strength and flexibility to structures throughout the body. In PXE, mineralization can affect elastic fibers in the skin, eyes, and blood vessels, and less frequently in other areas such as the digestive tract. People with PXE may have yellowish bumps called papules on their necks, underarms, and other areas of skin that touch when a joint bends (flexor areas). They may also have abnormalities in the eyes, such as a change in the pigmented cells of the retina (the light-sensitive layer of cells at the back of the eye) known as peau d'orange. Another eye abnormality known as angioid streaks occurs when tiny breaks form in the layer of tissue under the retina called Bruch's membrane. Bleeding and scarring of the retina may also occur, which can cause vision loss. Mineralization of the blood vessels that carry blood from the heart to the rest of the body (arteries) may cause other signs and symptoms of PXE. For example, people with this condition can develop narrowing of the arteries (arteriosclerosis) or a condition called claudication that is characterized by cramping and pain during exercise due to decreased blood flow to the arms and legs. Rarely, bleeding from blood vessels in the digestive tract may also occur. Frequency PXE affects approximately 1 in 50,000 people worldwide. For reasons that are unclear, this disorder is diagnosed twice as frequently in females as in males. -

ABCA12 Is the Major Harlequin Ichthyosis Gene Anna C
ORIGINAL ARTICLE ABCA12 Is the Major Harlequin Ichthyosis Gene Anna C. Thomas1, Tom Cullup2, Elizabeth E. Norgett1, Tara Hill3, Stephanie Barton4, Beverly A. Dale5, Eli Sprecher6, Eamonn Sheridan7, Aileen E. Taylor8, Robert S. Wilroy9, Celia DeLozier10, Nigel Burrows11, Helen Goodyear12, Philip Fleckman5, Karen G. Stephens5, Lakshmi Mehta13, Rosemarie M. Watson14, Robert Graham15, Roni Wolf16, Anne Slavotinek17, Madelena Martin17, David Bourn4, Charles A. Mein2, Edel A. O’Toole1 and David P. Kelsell1 Harlequin ichthyosis (HI) is the most severe form of autosomal-recessive, congenital ichthyosis. Affected infants have markedly impaired barrier function and are more susceptible to infection. Abnormalities in the localization of epidermal lipids as well as abnormal lamellar granule formation are features of HI skin. Previously, we and others have shown that mutations in the ABCA12 gene encoding an adenosine triphosphate-binding cassette (ABC) transporter underlie the skin disease HI. In this study, we have sequenced the ABCA12 gene in an additional 14 patients and show that all contain mutations, with the majority being either nonsense substitution or frameshift mutations. Eleven HI patients had bi-allelic ABCA12 mutations, whereas in the remaining three HI patients in this study, ABCA12 mutations were detected on only one allele by sequencing. In addition, the one patient from the previous study where no sequence mutations were detected was screened for heterozygous deletions. A combination of oligonucleotide arrays, multiplex PCR analysis -

Pseudoxanthoma Elasticum[Version 1; Peer Review: 1
F1000Research 2020, 9:9 Last updated: 16 JUL 2021 CASE REPORT Case Report: Pseudoxanthoma elasticum [version 1; peer review: 1 approved, 3 approved with reservations] Catarina Lucas 1, João Aranha2, Isabel da Rocha3, Domingos Sousa 4 1Family Health Unit, Baesuris Family Health Unit, Castro Marim, Faro, 8950-219, Portugal 2Dermatology Department, Hospital Distrital de Santarém, Santarém, Santarém, 2005-177, Portugal 3Personalized Health Care Unit, Personalized Health Care Unit of Penacova, Coimbra, Coimbra, 3360-205, Portugal 4Internal Medicine Department, Centro Hospitalar e Universitário do Algarve, Faro, Faro, 8000-386, Portugal v1 First published: 09 Jan 2020, 9:9 Open Peer Review https://doi.org/10.12688/f1000research.21431.1 Latest published: 09 Jan 2020, 9:9 https://doi.org/10.12688/f1000research.21431.1 Reviewer Status Invited Reviewers Abstract Pseudoxanthoma elasticum (PXE) is a rare inherited disorder, 1 2 3 4 characterised by a progressive mineralization and fragmentation of elastic fibres of the skin, retina and cardiovascular system. At an initial version 1 stage, the skin usually exhibits distinctive lesions and subsequently 09 Jan 2020 report report report report extra-dermal manifestations. The diagnosis is based on clinical manifestations, histological analysis of the lesions and genetic 1. Olivier M. Vanakker , Ghent University analysis. This is a case report of a 12-year-old child complaining of painless, Hospital, Ghent, Belgium mildly itchy yellow papules in the cervical region with 1 year of evolution. 2. Márta Medvecz , Semmelweis University, PXE is currently an incurable disease and has a favourable prognosis Budapest, Hungary when cardiovascular and retinal complications are prevented and monitored. 3. -

Genodermatoses
GENODERMATOSES Genodermatoses What’s new? Nigel P Burrows C Filaggrin mutations underlie ichthyosis vulgaris and are a risk factor for atopy including eczema, allergic sensitization, asthma, allergic rhinitis and peanut allergy Abstract C A new classification and nomenclature for ichthyoses was Genetic skin diseases encompass a spectrum from the common to the published in 2009, peeling skin syndromes which may be rare. It is important for the clinician to be alert to the possibility that confused for the milder subtypes of epidermolysis bullosa are the patient may be presenting for the first time with one or more features distinct genetic entities of a genetic disease so that appropriate investigation and counselling can C Emerging evidence that pseudoxanthoma elasticum is a meta- take place. Recent discoveries have helped the understanding of many of bolic disorder resulting in calcification of elastic fibres these disorders. A few common and important genodermatoses are high- C Mammalian target of rapamycin (mTOR) inhibitor therapies are lighted in this article. showing promise in tuberous sclerosis complex C Vascular anomalies on the skin may be the presenting feature of Keywords cancer syndromes; collagen; epidermolysis bullosa; filaggrin; inherited syndromes genodermatoses; ichthyosis; keratinization; pseudoxanthoma elasticum; vascular anomalies X-linked recessive ichthyosis In 75% of cases of X-linked recessive ichthyosis (XLRI) (Figure 1), scaling is present in the first week of life and tends to progress into adolescence. In contrast to IV, the flexures may be Genetic skin diseases encompass a spectrum from the common involved. A third of cases are associated with a prolonged labour. (e.g. atopic eczema) to the rare (e.g. -

Genetics of Lipedema: New Perspectives on Genetic Research and Molecular Diagnoses S
European Review for Medical and Pharmacological Sciences 2019; 23: 5581-5594 Genetics of lipedema: new perspectives on genetic research and molecular diagnoses S. PAOLACCI1, V. PRECONE2, F. ACQUAVIVA3, P. CHIURAZZI4,5, E. FULCHERI6,7, M. PINELLI3,8, F. BUFFELLI9,10, S. MICHELINI11, K.L. HERBST12, V. UNFER13, M. BERTELLI2; GENEOB PROJECT 1MAGI’S LAB, Rovereto (TN), Italy 2MAGI EUREGIO, Bolzano, Italy 3Department of Translational Medicine, Section of Pediatrics, Federico II University, Naples, Italy 4Istituto di Medicina Genomica, Fondazione A. Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy 5UOC Genetica Medica, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy 6Fetal and Perinatal Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy 7Department of Integrated Surgical and Diagnostic Sciences, University of Genoa, Genoa, Italy 8Telethon Institute of Genetics and Medicine (TIGEM), Pozzuoli, Italy 9Fetal and Perinatal Pathology Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy 10Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics and Maternal-Infantile Sciences, University of Genoa, Genoa, Italy 11Department of Vascular Rehabilitation, San Giovanni Battista Hospital, Rome, Italy 12Department of Medicine, University of Arizona, Tucson, AZ, USA 13Department of Developmental and Social Psychology, Faculty of Medicine and Psychology, Sapienza University of Rome, Rome, Italy Abstract. – OBJECTIVE: The aim of this quali- Introduction tative review is to provide an update on the cur- rent understanding of the genetic determinants of lipedema and to develop a genetic test to dif- Lipedema is an underdiagnosed chronic debil- ferentiate lipedema from other diagnoses. itating disease characterized by bruising and pain MATERIALS AND METHODS: An electronic and excess of subcutaneous adipose tissue of the search was conducted in MEDLINE, PubMed, and legs and/or arms in women during or after times Scopus for articles published in English up to of hormone change, especially in puberty1. -

Table I. Genodermatoses with Known Gene Defects 92 Pulkkinen
92 Pulkkinen, Ringpfeil, and Uitto JAM ACAD DERMATOL JULY 2002 Table I. Genodermatoses with known gene defects Reference Disease Mutated gene* Affected protein/function No.† Epidermal fragility disorders DEB COL7A1 Type VII collagen 6 Junctional EB LAMA3, LAMB3, ␣3, 3, and ␥2 chains of laminin 5, 6 LAMC2, COL17A1 type XVII collagen EB with pyloric atresia ITGA6, ITGB4 ␣64 Integrin 6 EB with muscular dystrophy PLEC1 Plectin 6 EB simplex KRT5, KRT14 Keratins 5 and 14 46 Ectodermal dysplasia with skin fragility PKP1 Plakophilin 1 47 Hailey-Hailey disease ATP2C1 ATP-dependent calcium transporter 13 Keratinization disorders Epidermolytic hyperkeratosis KRT1, KRT10 Keratins 1 and 10 46 Ichthyosis hystrix KRT1 Keratin 1 48 Epidermolytic PPK KRT9 Keratin 9 46 Nonepidermolytic PPK KRT1, KRT16 Keratins 1 and 16 46 Ichthyosis bullosa of Siemens KRT2e Keratin 2e 46 Pachyonychia congenita, types 1 and 2 KRT6a, KRT6b, KRT16, Keratins 6a, 6b, 16, and 17 46 KRT17 White sponge naevus KRT4, KRT13 Keratins 4 and 13 46 X-linked recessive ichthyosis STS Steroid sulfatase 49 Lamellar ichthyosis TGM1 Transglutaminase 1 50 Mutilating keratoderma with ichthyosis LOR Loricrin 10 Vohwinkel’s syndrome GJB2 Connexin 26 12 PPK with deafness GJB2 Connexin 26 12 Erythrokeratodermia variabilis GJB3, GJB4 Connexins 31 and 30.3 12 Darier disease ATP2A2 ATP-dependent calcium 14 transporter Striate PPK DSP, DSG1 Desmoplakin, desmoglein 1 51, 52 Conradi-Hu¨nermann-Happle syndrome EBP Delta 8-delta 7 sterol isomerase 53 (emopamil binding protein) Mal de Meleda ARS SLURP-1 -

Oral Manifestation of Genodermatoses L
Journal of Medicine, Radiology, Pathology & Surgery (2017), 4, 22–27 NARRATIVE REVIEW Oral manifestation of genodermatoses L. Kavya, S. Vandana, Swetha Paulose, Vishwanath Rangdhol, W. John Baliah, T. Dhanraj Department of Oral Medicine and Radiology, Indira Gandhi Institute of Dental Sciences, Pudhucherry, India Keywords Abstract Genodermatoses, mutation, oral Genodermatoses are inherited dermatological disorders associated with the structure manifestation and functions of skin and its appendages. Several genodermatoses presenting with Correspondence multisystem involvement lead to increased morbidity and mortality. Dermatological Dr. L. Kavya, Department of Oral Medicine and diseases, besides including the skin and its supplements may also involve the oral cavity, Radiology, Indira Gandhi Institute of Dental which deserves special attention considering that they may be the only presenting sign Sciences, Pudhucherry, India. of these disorders. The important aspect to be noted about these disorders is the rarity Phone: +91-9442902745. of the conditions and lack of awareness among the population which are the major E-mail: [email protected] drawbacks in the early diagnosis and prompt management of these diseases. This review intends to outline various genodermatoses with their characteristic oral manifestations. Received: 11 April 2017; Accepted: 15 May 2017 doi: 10.15713/ins.jmrps.97 Introduction hence, it is simplified under three classifications based on its distinct features. Genodermatoses or genetic diseases are a group of inherited According to William et al. 2005:[3] skin disorders with a collection of cutaneous and systemic signs 1. Chromosomal and symptoms. In the oral cavity, a wide spectrum of diseases 2. Single gene occurs due to genetic modifications ranging from developmental 3. -

Progress in Molecular Genetics of Heritable Skin Diseases: the Paradigms of Epidermolysis Bullosa and Pseudoxanthoma Elasticum
Progress in Molecular Genetics of Heritable Skin Diseases: The Paradigms of Epidermolysis Bullosa and Pseudoxanthoma Elasticum Jouni Uitto, Leena Pulkkinen, and Franziska Ringpfeil Departments of Dermatology and Cutaneous Biology, and Biochemistry and Molecular Pharmacology, Je¡erson Medical College, and Je¡erson Institute of Molecular Medicine,Thomas Je¡erson University, Philadelphia, Pennsylvania, U.S.A. The 42nd Annual Symposium on the Biology of the this meeting just caught the wave of early pioneering Skin, entitled ‘‘The Genetics of Skin Disease’’, was held studies that have helped us to understand the molecular in Snowmass Village, Colorado, in July 1993. That meet- basis of a large number of genodermatoses. This over- ing presented the opportunity to discuss how modern view presented in the 50th Annual Symposium on the approaches to molecular genetics and molecular biol- biology of the skin, highlights the progress made in ogy could be applied to understanding the mechanisms the molecular genetics of heritable skin diseases over of skin diseases. The published proceedings of this the past decade. Key words: Genodermatoses/epidermolysis meeting stated that ‘‘It is an opportune time to examine bullosa/pseudoxanthoma elasticum JID Symposium Proceed- the genetics of skin disease’’ (Norris et al, 1994). Indeed, ings 7:6^16,2002 he recent progress made in molecular genetics of the basis of clinical, histopathologic, immunohistochemical, and/ heritable skin diseases is abundantly evident from or ultrastructural analysis, to serve as candidate gene/protein sys- the present vantage point, as reviewed in the 50th tems. For example, in the case of EB, we initially postulated that Annual Montagna Symposium on the Biology of mutations in the structural genes expressed within the cutaneous Skin, also held in Snowmass Village, Colorado, in basement membrane zone (BMZ) could harbor mutations that TJuly 2001. -

1 – Ust-Dzhegutinsky District; 2
Supplementary Tables S1–S6 Note: 1 – Ust-Dzhegutinsky district; 2 – Karachaevsky district; 3 – Malokarachaevsky district; 4– Cherkessk City; 5 – Prikubansky district; 6 – Urupsky district; 7 – Zelenchuksky district; 8 – Abazinsky district; 9 – Khabezsky district; 10 – Adyge-Khablsky district; 11 – Nogaysky district; T/I – type of inheritance; AD – autosomal dominant type of inheritance, AR – autosomal recessive type of inheritance, XL – X-linked type of inheritance. PS – Phenotypic Series for OMIM in case of heterogeneity of the disease; Isol. – Isolated cases; Som. – Somatic mutation Table S1. Nosological spectrum and prevalence (per 100000) of hereditary neurological diseases in Karachay-Cherkess Republic (KChR) Number of patients Prevalence (per 100000) 1 2 3 4 5 6 7 8 9 10 11 Σ Kara Russi Circ Aba Nog Other Σ № ОМIМ Diagnosis T/I chays an assia zins ais s ns 1. #156200 Undifferentiated mental retardation АD 7 4 4 7 4 6 7 39 12.31 6.68 15.7 13.6 9.50 2. #249500 Undifferentiated mental retardation AR 10 10 19 6 23 9 1 2 17 16 20 133 31.40 26.71 41.2 9.02 74.6 76.68 32.41 3. #309530 Undifferentiated mental retardation XL 10 8 3 6 18 5 6 3 12 2 3 76 40.63 26.71 19.7 42.1 54.3 125.5 37.04 4. #300624 Martin-Bell syndrome XL 2 1 4 3 2 2 1 15 8.62 7.87 36.1 7.31 5. #158600 Spinal muscular atrophy, juvenile, proximal АD 2 2 1.48 0.49 6. #253300 Spinal muscular atrophy, type 1 AR 1 1 2 0.62 3.01 0.49 7. -

Excluded Conditions
Medical Diagnoses Not an Established Condition - 3 - Not an Established Condition 3-Methylcrotonyl-Coenzyme A (CoA) Carboxylase Deficiency 33-34 weeks EGA (if the birth weight is >1325 grams or 2 lbs 15 oz) 3rd Nerve Palsy (aka: Third Nerve Palsy; Third Cranial Nerve Palsy; 3rd Cranial Nerve Palsy; Oculomotor Palsy; Oculomotor Nerve Palsy; Cranial Nerve III Palsy) - 6 - Not an Established Condition 6th Nerve Palsy (aka: Abducens palsy; Lateral rectus palsy; Cranial mononeuropathy VI) - A - Not an Established Condition Aarskog-Scott syndrome Abnormal Neurological Exam at Discharge from NICU Absent Septum Pellucidum (aka: Absence Septum Pellucidum, Absent Cavum Septum Pellucidum) Achondroplasia Acute Lymphoid Leukemia (aka: ALL) Adams Oliver Syndrome (aka: AOS; Absence Defect of Limbs, Scalp, and Skull; Congenital Scalp Defects with Distal Limb Reduction Anomalies; Aplasia Cutis Congenita with Terminal Transverse Limb Defects) Adjustment Disorder (as defined within DC:0-3R, and diagnosed by specially-qualified professional) Alagille Syndrome (aka: AHD; Arteriohepatic Dysplasia; Cholestasis with Peripheral Pulmonary Stenosis; Syndromatic Hepatic Ductular Hypoplasia) Albinism (aka Ocular Cutaneous Albinism, Oculocutaneous Albinism, Ocular Albinism) Alcohol-Related Neurodevelopmental Disorder (aka: ARND; Fetal Alcohol Spectrum Disorder; FASD) Alport Syndrome (aka: Hematuria-Nephropathy Deafness; Hemorrhagic Familial Nephritis; Hereditary Deafness and Nephropathy; Hereditary Nephritis With Sensory Deafness; Hereditary Nephritis and Nerve Deafness)