Genodermatoses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bilateral Lower Extremity Hyperkeratotic Plaques: a Case Report of Ichthyosis Vulgaris
Faculty & Staff Scholarship 2015 Bilateral lower extremity hyperkeratotic plaques: a case report of ichthyosis vulgaris Hayley Leight Zachary Zinn Omid Jalali Follow this and additional works at: https://researchrepository.wvu.edu/faculty_publications Clinical, Cosmetic and Investigational Dermatology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Bilateral lower extremity hyperkeratotic plaques: a case report of ichthyosis vulgaris Hayley Leight Abstract: Here, we report a case of a middle-aged woman presenting with severe, long-standing, Zachary Zinn hyperkeratotic plaques of the lower extremities unrelieved by over-the-counter medications. Omid Jalali Initial history and clinical findings were suggestive of an inherited ichthyosis. Ichthyoses are genetic disorders characterized by dry scaly skin and altered skin-barrier function. A diagnosis Department of Dermatology, West Virginia University, of ichthyosis vulgaris was confirmed by histopathology. Etiology, prevalence, and treatment Morgantown, WV, USA options are discussed. Keywords: filaggrin gene, FLG, profilaggrin, keratohyalin granules, hyperkeratosis Introduction For personal use only. Inherited ichthyoses are a diverse group of genetic disorders characterized by dry, scaly skin; hyperkeratosis; and altered skin-barrier function. While these disorders of cutaneous keratinization are multifaceted and varying in etiology, disruption in the stratum corneum with generalized scaling is common to all.1–4 Although not entirely known -

Revertant Mosaicism in a Human Skin Fragility Disorder Results from Slipped Mispairing and Mitotic Recombination
Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi, … , Leena Bruckner-Tuderman, Cristina Has J Clin Invest. 2012;122(5):1742-1746. https://doi.org/10.1172/JCI61976. Brief Report Dermatology Spontaneous gene repair, also called revertant mosaicism, has been documented in several genetic disorders involving organs that undergo self-regeneration, including the skin. Genetic reversion may occur through different mechanisms, and in a single individual, the mutation can be repaired in various ways. Here we describe a disseminated pattern of revertant mosaicism observed in 6 patients with Kindler syndrome (KS), a genodermatosis caused by loss of kindlin-1 (encoded by FERMT1) and clinically characterized by patchy skin pigmentation and atrophy. All patients presented duplication mutations (c.456dupA and c.676dupC) in FERMT1, and slipped mispairing in direct nucleotide repeats was identified as the reversion mechanism in all investigated revertant skin spots. The sequence around the mutations demonstrated high propensity to mutations, favoring both microinsertions and microdeletions. Additionally, in some revertant patches, mitotic recombination generated areas with homozygous normal keratinocytes. Restoration of kindlin-1 expression led to clinically and structurally normal skin. Since loss of kindlin-1 severely impairs keratinocyte proliferation, we predict that revertant cells have a selective advantage that allows their clonal expansion and, consequently, the improvement of the skin condition. Find the latest version: https://jci.me/61976/pdf Brief report Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi,1 Yinghong He,1 Anna M.G. Pasmooij,2 Meltem Onder,3 Rudolf Happle,1 Marcel F. -

EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies
Focusing on Personalised Medicine EXTENDED CARRIER SCREENING Peace of Mind for Planned Pregnancies Extended carrier screening is an important tool for prospective parents to help them determine their risk of having a child affected with a heritable disease. In many cases, parents aren’t aware they are carriers and have no family history due to the rarity of some diseases in the general population. What is covered by the screening? Genomics For Life offers a comprehensive Extended Carrier Screening test, providing prospective parents with the information they require when planning their pregnancy. Extended Carrier Screening has been shown to detect carriers who would not have been considered candidates for traditional risk- based screening. With a simple mouth swab collection, we are able to test for over 419 genes associated with inherited diseases, including Fragile X Syndrome, Cystic Fibrosis and Spinal Muscular Atrophy. The assay has been developed in conjunction with clinical molecular geneticists, and includes genes listed in the NIH Genetic Test Registry. For a list of genes and disorders covered, please see the reverse of this brochure. If your gene of interest is not covered on our Extended Carrier Screening panel, please contact our friendly team to assist you in finding a gene test panel that suits your needs. Why have Extended Carrier Screening? Extended Carrier Screening prior to pregnancy enables couples to learn about their reproductive risk and consider a complete range of reproductive options, including whether or not to become pregnant, whether to use advanced reproductive technologies, such as preimplantation genetic diagnosis, or to use donor gametes. -

Harlequin Ichthyosis
orphananesthesia Anaesthesia recommendations for patients suffering from Harlequin ichthyosis Disease name: Harlequin ichthyosis ICD 10: Q80.4 Synonyms: Harlequin baby, ichthyosis congenita, Ichthyosis fetalis, keratosis diffusa fetalis, Harlequin fetus, Ichthyosis congenita gravior Disease summary: Harlequin ichthyosis (HI) is an autosomal recessive congenital ichthyosis. HI is an extremely rare and most severe form of ichthyosis. The condition is caused by mutation of the ABCA12 gene resulting in impaired lipid transport in the outermost layer of the skin, the epidermis. During the neontatal period, harlequin ichthyosis manifests phenotypically as dramatic large polygonal plate-like scaling of the skin that cracks and can slough, revealing the underlying diffusely bright red skin. These thick skin plates can pull and distort facial features. The tightness of the skin can also pull on the eyes and mouth resulting in difficulties with closing these structures. The tightness also causes the eyes and the mouth to turn inside out resulting in ectropion and eclabium. Other features include hypoplasia of the fingers, malformation of the ears and nose, and alopecia. Affected neonates often do not survive and mortality is commonly attributed to respiratory failure and/or sepsis. Clinical data obtained from 45 HI patients revealed 25 survivors and 20 deaths with an overall survival rate of only 56%. The ages of survivors ranged from 10 months to 25 years and death usually occurred in the first 3 months. HI infants need to be cared for in a neonatal intensive care unit immediately after birth. Several harlequin neonates have survived. They tend to have severe erythroderma and fine scaling, even with optimal management. -

RD-Action Matchmaker – Summary of Disease Expertise Recorded Under
Summary of disease expertise recorded via RD-ACTION Matchmaker under each Thematic Grouping and EURORDIS Members’ Thematic Grouping Thematic Reported expertise of those completing the EURORDIS Member perspectives on Grouping matchmaker under each heading Grouping RD Thematically Rare Bone Achondroplasia/Hypochondroplasia Achondroplasia Amelia skeletal dysplasia’s including Achondroplasia/Growth hormone cleidocranial dysostosis, arthrogryposis deficiency/MPS/Turner Brachydactyly chondrodysplasia punctate Fibrous dysplasia of bone Collagenopathy and oncologic disease such as Fibrodysplasia ossificans progressive Li-Fraumeni syndrome Osteogenesis imperfecta Congenital hand and fore-foot conditions Sterno Costo Clavicular Hyperostosis Disorders of Sex Development Duchenne Muscular Dystrophy Ehlers –Danlos syndrome Fibrodysplasia Ossificans Progressiva Growth disorders Hypoparathyroidism Hypophosphatemic rickets & Nutritional Rickets Hypophosphatasia Jeune’s syndrome Limb reduction defects Madelung disease Metabolic Osteoporosis Multiple Hereditary Exostoses Osteogenesis imperfecta Osteoporosis Paediatric Osteoporosis Paget’s disease Phocomelia Pseudohypoparathyroidism Radial dysplasia Skeletal dysplasia Thanatophoric dwarfism Ulna dysplasia Rare Cancer and Adrenocortical tumours Acute monoblastic leukaemia Tumours Carcinoid tumours Brain tumour Craniopharyngioma Colon cancer, familial nonpolyposis Embryonal tumours of CNS Craniopharyngioma Ependymoma Desmoid disease Epithelial thymic tumours in -
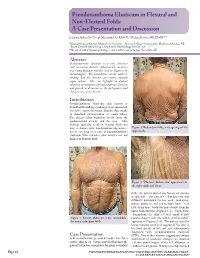
Pseudoxanthoma Elasticum in Flexural and Non-Flexural Folds: a Case Presentation and Discussion
Pseudoxanthoma Elasticum in Flexural and Non-Flexural Folds: A Case Presentation and Discussion Shahrzad Akbary, BS,* Joseph Machuzak, DO, FAOCD,** Richard Bernert, MD, FASDP*** *Medical Student, 4th year, Midwestern University - Arizona College of Osteopathic Medicine, Glendale, AZ **Board Certified Dermatologist, MacKenzie Dermatology, Prescott, AZ ***Board Certified Dermatopathologist, Arizona Dermatopathology, Scottsdale, AZ Abstract Pseudoxanthoma elasticum is a rare, inherited connective-tissue disorder. Characteristic cutaneous and biopsy findings typically lend to diagnosis by dermatologists. The presentation can be subtle or striking, and the disorder can involve multiple organ systems. Here we highlight an atypical cutaneous presentation of pseudoxanthoma elasticum and provide a discussion on the pathogenesis and characteristics of the disorder. PseudoxanthomaIntroduction elasticum, also known as Grönblad-Strandberg syndrome, is an autosomal- recessive connective-tissue disorder that results in abnormal mineralization of elastic fibers. The disease often manifests in the skin, the cardiovascular system, and the eyes. Skin findings typically occur in flexural folds and have a characteristic xanthomatous appearance; Figure 2. Redundant folds; note sparing of the herein, we present a case of pseudoxanthoma upper back. elasticum with extensive skin involvement not limited to flexural folds. Figure 3. “Plucked chicken skin” appearance in the right axilla and chest. folds; the patient denied any history of extreme weight loss. The patient’s redundant skin was diffusely distributed to his neck, underarms, thorax, abdomen, and mid to lower back. Very little of his upper body was spared aside from his upper back and face (Figures 1, 2). Upon closer examination, the skin revealed small yellow Figure 1. Thorax, abdomen, arms; remarkable papules characteristic of a “plucked chicken skin” for many redundant folds. -

Pseudoxanthoma Elasticum
Pseudoxanthoma elasticum Description Pseudoxanthoma elasticum (PXE) is a progressive disorder that is characterized by the accumulation of deposits of calcium and other minerals (mineralization) in elastic fibers. Elastic fibers are a component of connective tissue, which provides strength and flexibility to structures throughout the body. In PXE, mineralization can affect elastic fibers in the skin, eyes, and blood vessels, and less frequently in other areas such as the digestive tract. People with PXE may have yellowish bumps called papules on their necks, underarms, and other areas of skin that touch when a joint bends (flexor areas). They may also have abnormalities in the eyes, such as a change in the pigmented cells of the retina (the light-sensitive layer of cells at the back of the eye) known as peau d'orange. Another eye abnormality known as angioid streaks occurs when tiny breaks form in the layer of tissue under the retina called Bruch's membrane. Bleeding and scarring of the retina may also occur, which can cause vision loss. Mineralization of the blood vessels that carry blood from the heart to the rest of the body (arteries) may cause other signs and symptoms of PXE. For example, people with this condition can develop narrowing of the arteries (arteriosclerosis) or a condition called claudication that is characterized by cramping and pain during exercise due to decreased blood flow to the arms and legs. Rarely, bleeding from blood vessels in the digestive tract may also occur. Frequency PXE affects approximately 1 in 50,000 people worldwide. For reasons that are unclear, this disorder is diagnosed twice as frequently in females as in males. -

Fetal Harlequin Ichthyosis – a Case Report
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 14, Issue 11 Ver. I (Nov. 2015), PP 81-86 www.iosrjournals.org Fetal Harlequin Ichthyosis – A Case Report Sunita Nayak1, Suren Prasad Dash2, Muktikanta Khatua3 1Tutor, Department of Anatomy, SCB Medical College, Cuttack, Odisha, India 2Consultant Radiologist, Sahara Diagnostics, Courtpeta Square, Berhampur, Odisha, India 3Assistant Surgeon, District Headquarter Hospital, Koraput, Odisha, India Abstract: Ichthyosis refers to a relatively uncommon group of skin disorders characterized by the presence of excessive amounts of dry surface scales. Autosomal recessive congenital ichthyosis (ARCI) encompasses several forms of nonsyndromic ichthyosis. Although most neonates with ARCI are collodion babies, the clinical presentation and severity of ARCI may vary significantly, ranging from harlequin ichthyosis, the most severe and often fatal form, to lamellar ichthyosis (LI) and (nonbullous) congenital ichthyosiform erythroderma (CIE). This is a case report of congenital (Harlequin) ichthyosis which is also called harlequin fetus, a lethal keratinising disorder. An externally thickened keratin layer of skin and diffuse plate like scales characterize it. Prenatal sonographic diagnosis has been described, with 2D findings of a persistently open mouth, echogenic amniotic fluid and fixed flexion deformity of the extremities. The 3D sonographic features have been described showing the morphological appearance typical of harlequin fetus, namely the open mouth with thick lips. The most definitive prenatal sonographic diagnosis of this condition is by 3D, which may not be done routinely without a suspicious 2D feature. In this case report, we suggest that the appearance of facial profile is the most easily seen and earliest 2D feature of this condition. -

Proceedings of the 16Th Annual Meeting of the Society for Pediatric Dermatoiogy
SPECIAL ARTICLE Pediatric Dermatology Vol. 9 No. 1 66-76 Proceedings of the 16th Annual Meeting of the Society for Pediatric Dermatoiogy WiUiamsburg, Virginia June 3a-July 3, 1991 Eleanor £. Sahn, M.D. Medical University of South Carolina Charleston, South Carolina A. Howiand Hartley, M.D. Children's Hospital National Medical Center Washington, D.C. Stephen Gellis, M.D. Children's Hospital Medical Center Boston, Massachusetts James E. Rasmussen, M.D. University of Michigan Medical Center Ann Arbor, Michigan Monday, July 1, 1991 ture by the newspaper account he received, dated December 17, 1799, telling of General George Dr. Alfred T. Lane (Stanford University) orga- Washington's death. We learn the story of General nized the sixteenth annual meeting of the Society Washington's rapid demise, probably from bacterial for Pediatric Dermatology, held in Wiliiamsburg, infection, hastened by the medical treatments of the Virgitiia. The seventh annual Sidney Hurwitz Lec- day, including frequent and copious blood letting. ture was delivered by Dr. Rona M. MacKie (Uni- There was a current saying, "more people died an- versity of Glasgow) on "Melanoma: Risk Factors in nually from lancets than from swords." Dysplastic Nevus Syndrome." President Anne Lucky (Cincinnati, Ohio) welcomed the society MELANOMA: RISK FACTORS AND members to Wiliiamsburg and introduced the first DYSPLASTIC NEVUS SYNDROME speaker. Dr. Rona MacKie first discussed risk factors in mel- anoma, citing several large case control studies car- COLONIAL MEDICINE ried out in western Canada, Scotland, Scandinavia, Dr. Tor A. Shwayder (Henry Ford Hospital) pre- and Germany. The frequency of melanoma has dou- sented a delightful and professional "Character In- bled each decade in Scandinavia, the United King- terpreter Portrayal of Iseiac Shwayder, Medical dom, and Germany. -

Mutation and Expression of Abca12in Keratosis Pilaris and Nevus
MOLECULAR MEDICINE REPORTS 18: 3153-3158, 2018 Mutation and expression of ABCA12 in keratosis pilaris and nevus comedonicus FEN LIU1,2*, YAO YANG1,3*, YAN ZHENG1,3, YAN-HUA LIANG1,3 and KANG ZENG1 1Department of Dermatology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong 510515; 2Department of Histology and Embryology, Institute of Neuroscience, School of Basic Medical Sciences, Wenzhou Medical University, Wenzhou, Zhejiang 325035; 3Department of Dermatology, Shenzhen Hospital, Southern Medical University, Shenzhen, Guangdong 518100, P.R. China Received June 22, 2017; Accepted April 17, 2018 DOI: 10.3892/mmr.2018.9342 Abstract. Keratosis pilaris (KP) and nevus comedonicus Introduction (NC) are congenital keratinized dermatoses; however, the exact etiology of these two diseases is unclear. The objective Keratosis pilaris (KP; OMIM #604093), also known as of the present study was to identify the disease-causing genes lichen pilaris, is a benign genodermatosis that is estimated and their association with functional alterations in the devel- to effect ~40% of the population (1). KP is characterized by opment of KP and NC. Peripheral blood samples of one KP the presence of symmetric, asymptomatic and grouped kera- family, two NC families and 100 unrelated healthy controls totic follicular papules with varying degrees of perifollicular were collected. The genomic sequences of 147 genes associ- erythema. KP lesions often involve the proximal and extended ated with 143 genetic skin diseases were initially analyzed parts of extremities, the cheeks and the buttocks (2). Cases from the KP proband using a custom-designed GeneChip. A may be generalized or unilateral (2). Most patients develop KP novel heterozygous missense mutation in the ATP-binding in their childhood, with a peak in incidence during adoles- cassette sub-family A member 12 (ABCA12) gene, designated cence (3). -

ABCA12 Is the Major Harlequin Ichthyosis Gene Anna C
ORIGINAL ARTICLE ABCA12 Is the Major Harlequin Ichthyosis Gene Anna C. Thomas1, Tom Cullup2, Elizabeth E. Norgett1, Tara Hill3, Stephanie Barton4, Beverly A. Dale5, Eli Sprecher6, Eamonn Sheridan7, Aileen E. Taylor8, Robert S. Wilroy9, Celia DeLozier10, Nigel Burrows11, Helen Goodyear12, Philip Fleckman5, Karen G. Stephens5, Lakshmi Mehta13, Rosemarie M. Watson14, Robert Graham15, Roni Wolf16, Anne Slavotinek17, Madelena Martin17, David Bourn4, Charles A. Mein2, Edel A. O’Toole1 and David P. Kelsell1 Harlequin ichthyosis (HI) is the most severe form of autosomal-recessive, congenital ichthyosis. Affected infants have markedly impaired barrier function and are more susceptible to infection. Abnormalities in the localization of epidermal lipids as well as abnormal lamellar granule formation are features of HI skin. Previously, we and others have shown that mutations in the ABCA12 gene encoding an adenosine triphosphate-binding cassette (ABC) transporter underlie the skin disease HI. In this study, we have sequenced the ABCA12 gene in an additional 14 patients and show that all contain mutations, with the majority being either nonsense substitution or frameshift mutations. Eleven HI patients had bi-allelic ABCA12 mutations, whereas in the remaining three HI patients in this study, ABCA12 mutations were detected on only one allele by sequencing. In addition, the one patient from the previous study where no sequence mutations were detected was screened for heterozygous deletions. A combination of oligonucleotide arrays, multiplex PCR analysis -

Dumitras, Cu, MC; Popa, A.; Petca, A.; Miulescu, R.-G. Cutaneous Mastocytosis in Childhood—Update from the Literature
Journal of Clinical Medicine Review Cutaneous Mastocytosis in Childhood—Update from the Literature 1,2 1,3 1,4 1,5, Florica Sandru ,Răzvan-Cosmin Petca , Monica Costescu , Mihai Cristian Dumitras, cu *, Adelina Popa 2, Aida Petca 1,6,* and Raluca-Gabriela Miulescu 1,7 1 “Carol Davila” University of Medicine and Pharmacy, 030167 Bucharest, Romania; fl[email protected] (F.S.); [email protected] (R.-C.P.); [email protected] (M.C.); [email protected] (R.-G.M.) 2 Department of Dermatology, Elias University Emergency Hospital, 0611461 Bucharest, Romania; [email protected] 3 Department of Urology, “Prof. Dr. Theodor Burghele” Clinical Hospital, 061344 Bucharest, Romania 4 Department of Dermatology, “Dr. Victor Babes” Clinical Hospital of Infectious and Tropical Diseases, 030303 Bucharest, Romania 5 Department of Obstetrics & Gynecology, University Emergency Hospital, 050098 Bucharest, Romania 6 Department of Obstetrics & Gynecology, Elias University Emergency Hospital, 0611461 Bucharest, Romania 7 Department of Dermatology, Vălenii de Munte Hospital, 106400 Prahova, Romania * Correspondence: [email protected] (M.C.D.); [email protected] (A.P.); Tel.: +40-722-223223 (M.C.D.); +40-745-787448 (A.P.) Abstract: Mastocytosis (M) represents a systemic pathology characterized by increased accumulation and clonal proliferation of mast cells in the skin and/or different organs. Broadly, M is classified into two categories: Cutaneous mastocytosis (CM) and systemic mastocytosis (SM). In children, CM is Citation: Sandru, F.; Petca, R.-C.; the most frequent form. Unfortunately, pathogenesis is still unclear. It is thought that genetic factors Costescu, M.; Dumitras, cu, M.C.; are involved, but further studies are necessary. As for features of CM, the lesions differ in clinical Popa, A.; Petca, A.; Miulescu, R.-G.