9 the Holometabola
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aquatic Insects Are Dramatically Underrepresented in Genomic Research
insects Communication Aquatic Insects Are Dramatically Underrepresented in Genomic Research Scott Hotaling 1,* , Joanna L. Kelley 1 and Paul B. Frandsen 2,3,* 1 School of Biological Sciences, Washington State University, Pullman, WA 99164, USA; [email protected] 2 Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT 84062, USA 3 Data Science Lab, Smithsonian Institution, Washington, DC 20002, USA * Correspondence: [email protected] (S.H.); [email protected] (P.B.F.); Tel.: +1-(828)-507-9950 (S.H.); +1-(801)-422-2283 (P.B.F.) Received: 20 August 2020; Accepted: 3 September 2020; Published: 5 September 2020 Simple Summary: The genome is the basic evolutionary unit underpinning life on Earth. Knowing its sequence, including the many thousands of genes coding for proteins in an organism, empowers scientific discovery for both the focal organism and related species. Aquatic insects represent 10% of all insect diversity, can be found on every continent except Antarctica, and are key components of freshwater ecosystems. However, aquatic insect genome biology lags dramatically behind that of terrestrial insects. If genomic effort was spread evenly, one aquatic insect genome would be sequenced for every ~9 terrestrial insect genomes. Instead, ~24 terrestrial insect genomes have been sequenced for every aquatic insect genome. A lack of aquatic genomes is limiting research progress in the field at both fundamental and applied scales. We argue that the limited availability of aquatic insect genomes is not due to practical limitations—small body sizes or overly complex genomes—but instead reflects a lack of research interest. We call for targeted efforts to expand the availability of aquatic insect genomic resources to empower future research. -

Viewed Erature to Ensure the Most Up-To-Date Treatment with Caution, P~Rticularlyamong Older Literature
PROCEEDINGS OF THE CALIFORNIA ACADEMY OF SCIENCES Vol. 50, No. 3, pp. 39-114. December 9, 1997 SPECIES CATALOG OF THE NEUROPTERA, MEGALOPTERA, AND RAPHIDIOPTERA OF AMERlCA NORTH OF MEXICO Norman D. Penny Department ofE~ztorizolog)~,Caldornla Acndony oJ'Sc~erzces, San Fmnc~sco,CA 941 18 Phillip A. Adams Ccllg'rnia State Utzivet-sity, F~lllet-ton,CA 92634 and Lionel A. Stange Florida Depat>tnzen/oj'Agt.~czi/trrre, Gr~~nesv~/le, FL 32602 Thc 399 currently recognized valid species of the orders Neuroptera, Megaloptera, and Raphidioptera that are known to occur in America north of Mexico are listed and full synonymies given. Geographical distributions are listed by states and province\. Complete bibliographic references are given for all namcs and nomenclatural acts. Included are two new Junior homonyms indicated, seven new taxonomic cornbinations, two new changes of rank, fourteen new synonymies, three new lectotype de\ignations, and onc new name. Received March 20,1996. Accepted June 3, 1997. The recent publication of Nomina Insecta been consulted whenever possible, as well as Nearctica, A Check List of the Insects of North Zoological Record, and appropriate mono- America (Poole 1996) has given us a listing of graphic revisions publishedup to 1 January 1997. North American Neuropterida (Neuroptera + A number of taxonomic changes are incorpo- Megaloptera + Raphidioptera) species for the rated into this catalog: there are two new Junior first tlme in more than a century. However, for homonyms indicated, seven new taxonomic anyone trying to identify these species, the litera- combinations, two new changes of rank. fourteen ture is scattered and obscure. -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

Megaloptera, Sialidae)
MUSEUM & INSTITUTE OF ZOOLOGY POLISH ACADEMY OF SCIENCES FRAGMENTA FAUN I STIC A Fragm. faun. Warsaw, 30.12.2000 43 11 123-125 Wiesława C z e c h o w s k a Sialis morio K lingstedt, 1932 Megaloptera( , S ia lid a), e an alderfly species new to Poland Abstract: Sialis morio K lingstedt, 1932 is reported from Poland for the first time. It was found in two sites in the Masurian Lake District in the years 1998-1999. Key words:Neuropteroidea, Megaloptera , Sialis morio, Poland. Author's address: Museum and Institute of Zoology, PAS, Wilcza 64, 00-679 Warszawa, POLAND The Megaloptera is a small order of insects of the superorder Neuropteroi dea whose larval development occurs in an aquatic habitat. In Europe, this taxon is represented by 10 species of the genus Sialis L a t r . , the family Siali dae (A s p ó c k et al. 1980, V s h iv k o v a 1985, 1987). However, according to A s p ó c k (1992) and A s p ó c k and H o l z e l (1994), this genus should be revised, for some of the recently described species may be synonyms of others. The species considered unquestionable by these authors include Sialis lutaria L., S. morio K l i n g s t . , S. sordida K l i n g s t . , S. fuliginosa PICT., S. rtigripes PICT, and S. sibirica M c L a c h l . Three of these have been recorded from Poland, namely S. -

A New Type of Neuropteran Larva from Burmese Amber
A 100-million-year old slim insectan predator with massive venom-injecting stylets – a new type of neuropteran larva from Burmese amber Joachim T. haug, PaTrick müller & carolin haug Lacewings (Neuroptera) have highly specialised larval stages. These are predators with mouthparts modified into venominjecting stylets. These stylets can take various forms, especially in relation to their body. Especially large stylets are known in larva of the neuropteran ingroups Osmylidae (giant lacewings or lance lacewings) and Sisyridae (spongilla flies). Here the stylets are straight, the bodies are rather slender. In the better known larvae of Myrmeleontidae (ant lions) and their relatives (e.g. owlflies, Ascalaphidae) stylets are curved and bear numerous prominent teeth. Here the stylets can also reach large sizes; the body and especially the head are relatively broad. We here describe a new type of larva from Burmese amber (100 million years old) with very prominent curved stylets, yet body and head are rather slender. Such a combination is unknown in the modern fauna. We provide a comparison with other fossil neuropteran larvae that show some similarities with the new larva. The new larva is unique in processing distinct protrusions on the trunk segments. Also the ratio of the length of the stylets vs. the width of the head is the highest ratio among all neuropteran larvae with curved stylets and reaches values only found in larvae with straight mandibles. We discuss possible phylogenetic systematic interpretations of the new larva and aspects of the diversity of neuropteran larvae in the Cretaceous. • Key words: Neuroptera, Myrmeleontiformia, extreme morphologies, palaeo evodevo, fossilised ontogeny. -

From Chewing to Sucking Via Phylogeny—From Sucking to Chewing Via Ontogeny: Mouthparts of Neuroptera
Chapter 11 From Chewing to Sucking via Phylogeny—From Sucking to Chewing via Ontogeny: Mouthparts of Neuroptera Dominique Zimmermann, Susanne Randolf, and Ulrike Aspöck Abstract The Neuroptera are highly heterogeneous endopterygote insects. While their relatives Megaloptera and Raphidioptera have biting mouthparts also in their larval stage, the larvae of Neuroptera are characterized by conspicuous sucking jaws that are used to imbibe fluids, mostly the haemolymph of prey. They comprise a mandibular and a maxillary part and can be curved or straight, long or short. In the pupal stages, a transformation from the larval sucking to adult biting and chewing mouthparts takes place. The development during metamorphosis indicates that the larval maxillary stylet contains the Anlagen of different parts of the adult maxilla and that the larval mandibular stylet is a lateral outgrowth of the mandible. The mouth- parts of extant adult Neuroptera are of the biting and chewing functional type, whereas from the Mesozoic era forms with siphonate mouthparts are also known. Various food sources are used in larvae and in particular in adult Neuroptera. Morphological adaptations of the mouthparts of adult Neuroptera to the feeding on honeydew, pollen and arthropods are described in several examples. New hypoth- eses on the diet of adult Nevrorthidae and Dilaridae are presented. 11.1 Introduction The order Neuroptera, comprising about 5820 species (Oswald and Machado 2018), constitutes together with its sister group, the order Megaloptera (about 370 species), and their joint sister group Raphidioptera (about 250 species) the superorder Neuropterida. Neuroptera, formerly called Planipennia, are distributed worldwide and comprise 16 families of extremely heterogeneous insects. -

Insects and Related Arthropods Associated with of Agriculture
USDA United States Department Insects and Related Arthropods Associated with of Agriculture Forest Service Greenleaf Manzanita in Montane Chaparral Pacific Southwest Communities of Northeastern California Research Station General Technical Report Michael A. Valenti George T. Ferrell Alan A. Berryman PSW-GTR- 167 Publisher: Pacific Southwest Research Station Albany, California Forest Service Mailing address: U.S. Department of Agriculture PO Box 245, Berkeley CA 9470 1 -0245 Abstract Valenti, Michael A.; Ferrell, George T.; Berryman, Alan A. 1997. Insects and related arthropods associated with greenleaf manzanita in montane chaparral communities of northeastern California. Gen. Tech. Rep. PSW-GTR-167. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Dept. Agriculture; 26 p. September 1997 Specimens representing 19 orders and 169 arthropod families (mostly insects) were collected from greenleaf manzanita brushfields in northeastern California and identified to species whenever possible. More than500 taxa below the family level wereinventoried, and each listing includes relative frequency of encounter, life stages collected, and dominant role in the greenleaf manzanita community. Specific host relationships are included for some predators and parasitoids. Herbivores, predators, and parasitoids comprised the majority (80 percent) of identified insects and related taxa. Retrieval Terms: Arctostaphylos patula, arthropods, California, insects, manzanita The Authors Michael A. Valenti is Forest Health Specialist, Delaware Department of Agriculture, 2320 S. DuPont Hwy, Dover, DE 19901-5515. George T. Ferrell is a retired Research Entomologist, Pacific Southwest Research Station, 2400 Washington Ave., Redding, CA 96001. Alan A. Berryman is Professor of Entomology, Washington State University, Pullman, WA 99164-6382. All photographs were taken by Michael A. Valenti, except for Figure 2, which was taken by Amy H. -
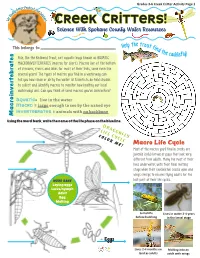
Creek Critters!
Grades 3-6 Creek Critter Activity Page 1 ter Protect Ot Ou lly r W ea a R t e e r W ! Creek Critters! Science With Spokane County Water Resources Help the trout f This belongs to: ind the ca ly! Fish, like the Redband Trout, eat aquatic bugs known as AQUATIC ddisf MACROINVERTEBRATES (macros for short). Macros live at the bottom of streams, rivers and lakes for most of their lives; some even live several years! The types of macros you find in a waterway can tell you how clean or dirty the water is! Scientists do field studies to collect and identify macros to monitor how healthy our local waterways are. Can you think of some macros you’ve seen before? Aquatic= live in the water Macro = large enough to see by the naked eye invertebrates = animals with no backbone Macroinvertebrates Using the word bank, write the name of the life phase on the blue line. DRAGONFLY LIFE CYCLE COLOR ME! Macro Life Cycle Most of the macros you’ll find in creeks are juvenile (child) larvae or pupa that look very different from adults. Many live most of their lives underwater, until their final molting stage when their exoskeleton cracks open and wings emerge to become flying adults for the Word Bank last part of their life cycles. Laying eggs Larva/nymph Adult Egg Molting 6 months Lives in water 3-4 years before hatching in the larval stage Eggs Lives 2-4 months on Molting into an land as adults adult with wings Grades 3-6 Creek Critter Activity Page 2 Table Manners! Freshwater Food Chain Macros have specialized mouth pieces to help them gather food or hunt. -

THE ORIGIN and EVOLUTION of HYMENOPTEROUS INSECTS [P
THE ORIGIN AND EVOLUTION OF HYMENOPTEROUS INSECTS [p. 24] Chapter 3 EVOLUTION OF THE INFRACLASS SCARABAEONES A. P. Rasnitzyn* Dragonflies [order Odonata] and mayflies [order Ephemeroptera], which have retained a primitive thorax structure (absence of a cryptosternum) but are specialized in other respects, occupy the most isolated position in the infraclass. They are similar to each other in a number of characters, but the nature of the latter does not allow drawing a conclusion about any close relationship of the two orders. Indeed, the primitiveness of the structure of the thorax common to both of them, like any symplesiomorphy, is not evidence of a common origin. The adaptations of the larvae of mayflies and dragonflies to an aquatic way of life are different, and they show, rather, an independent transition to development in water. The loss of the ability to fold the wings as well is connected with different processes in them: in mayflies, with the ephemerality of the imago, the function of which is essentially limited to nuptial flight and oviposition; and in dragonflies, with the metamorphosis of the adult insect to an active aerial predator. It is highly probable that mayflies and dragonflies are independent evolutionary branches. The belonging of dragonflies and mayflies to a common trunk of Scarabaeones, that is, the isolation of them from Protoptera as part of a single trunk with the remaining members of the infraclass (except Protoptera), is confirmed by the metamorphosis of the style of the ninth segment in mayfly males into part of the copulatory organ [endophallus], and in dragonfly females into a sheath of the ovipositor. -

Phylum Arthropoda*
Zootaxa 3703 (1): 017–026 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.6 http://zoobank.org/urn:lsid:zoobank.org:pub:FBDB78E3-21AB-46E6-BD4F-A4ADBB940DCC Phylum Arthropoda* ZHI-QIANG ZHANG New Zealand Arthropod Collection, Landcare Research, Private Bag 92170, Auckland, New Zealand; [email protected] * In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa, 3703, 1–82. Abstract The Arthropoda is here estimated to have 1,302,809 described species, including 45,769 fossil species (the diversity of fossil taxa is here underestimated for many taxa of the Arthropoda). The Insecta (1,070,781 species) is the most successful group, and it alone accounts for over 80% of all arthropods. The most successful insect order, Coleoptera (392,415 species), represents over one-third of all species in 39 insect orders. Another major group in Arthropoda is the class Arachnida (114,275 species), which is dominated by the Acari (55,214 mite and tick species) and Araneae (44,863 spider species). Other diverse arthropod groups include Crustacea (73,141 species), Trilobitomorpha (20,906 species) and Myriapoda (12,010 species). Key words: Classification, diversity, Arthropoda Introduction The Arthropoda, with over 1.5 million described species, is the largest animal phylum, and it alone accounts for about 80% of the total number of species in the animal kingdom (Zhang 2011a). In the last volume on animal higher-level classification and survey of taxonomic richness, 28 chapters by numerous teams of specialists were published on various taxa of the Arthropoda, but there were many gaps to be filled (Zhang 2011b). -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Evolution of the Insects David Grimaldi and Michael S
Cambridge University Press 0521821495 - Evolution of the Insects David Grimaldi and Michael S. Engel Frontmatter More information EVOLUTION OF THE INSECTS Insects are the most diverse group of organisms to appear in the 3-billion-year history of life on Earth, and the most ecologically dominant animals on land. This book chronicles, for the first time, the complete evolutionary history of insects: their living diversity, relationships, and 400 million years of fossils. Whereas other volumes have focused on either living species or fossils, this is the first comprehensive synthesis of all aspects of insect evolution. Current estimates of phylogeny are used to interpret the 400-million-year fossil record of insects, their extinctions, and radiations. Introductory sections include the living species, diversity of insects, methods of reconstructing evolutionary relationships, basic insect structure, and the diverse modes of insect fossilization and major fossil deposits. Major sections cover the relationships and evolution of each order of hexapod. The book also chronicles major episodes in the evolutionary history of insects: their modest beginnings in the Devonian, the origin of wings hundreds of millions of years before pterosaurs and birds, the impact that mass extinctions and the explosive radiation of angiosperms had on insects, and how insects evolved the most complex societies in nature. Evolution of the Insects is beautifully illustrated with more than 900 photo- and electron micrographs, drawings, diagrams, and field photographs, many in full color and virtually all original. The book will appeal to anyone engaged with insect diversity: professional ento- mologists and students, insect and fossil collectors, and naturalists. David Grimaldi has traveled in 40 countries on 6 continents collecting and studying recent species of insects and conducting fossil excavations.