Neuropterida of the Lower Cretaceous of Southern England, with a Study on Fossil and Extant Raphidioptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuroptera: Coniopterygidae) of the Arabian Peninsula
FAUNA OF ARABIA 22: 381-434 Date of publication: 18.12.2006 The dusty lacewings (Neuroptera: Coniopterygidae) of the Arabian Peninsula Gyorgy Sziraki and Antonius van Harten A b s t r act: The descriptions of nine new coniopterygid species (Cryptoscenea styfaris n. sp., Coniopteryx (Xeroconiopteryx) pfat yarcus n. sp., C (X) caudata n. sp., C (X) dudichi n. sp., C (X) stylobasalis n. sp., C (X) armata n. sp., C (X) loksai n. sp., C (Coniopteryx) gozmanyi n. sp., Conwentzia obscura n. sp.), and an annotated list of 53 other species of dusty lacewings found in the Arabian Peninsula are given, together with an identification key. Nine described species (Aleuropteryx wawrikae, Coniocompsa smithersi, Nimboa espanoli, N. kasyi, N. ressii, Coniopteryx (X) aegyptiaca, C (X) hastata, C (X) kerzhneri, C (X) mongolica) are also new to the fauna of the Arabian Peninsula. Aleuropteryx cruciata Szid.ki, 1990 is regarded as a junior synonym of A. arabica Meinander, 1977, while Helicoconis serrata Meinander, 1979 is transferred to the genus Cryptoscenea. A new informal species-group (the unguihipandriata-group) is proposed within the subgenus Xeroconiopteryx. Coniopteryx (X) martinmeinanderi nom. nov. is pro posed for C (X) forcata Meinander, 1998, which is a junior primary homonym. 4.~, 0.fi?--' ~ J (Coniopterygidae :~~, ~) o~' ~~, ~~) ~ ~ I! .. ~ :; J.jJpl ;y..ill ~":il 4J.~j if ~y of' ~ ~ WLi ~JJ t.lyl a.........;.....A..PJ f' :~~ 41"';"'i ~ ~ ;~..b:- t.lyi L4i J.>- J.j.J4' y t.'f! a.........; j ~i f' .~ c.l:A.. Jl ~W,l ,~..rJI ; .;:!y.,.1 Helicoconis -

Ent19 1 003 008 (Moseiko).Pmd
Russian Entomol. J. 19(1): 38 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2010 Medvedemolpus gen.n. a new genus of Eumolpinae (Coleoptera: Chrysomelidae) from Philippines Medvedemolpus gen.n. íîâûé ðîä Eumolpinae (Coleoptera: Chrysomelidae) ñ Ôèëèïïèí A.G. Moseyko À.Ã. Ìîñåéêî Zoological Institute of Russian Academy of Sciences, Universitetskaya nab., 1, St. Petersburg 199034, Russia. Çîîëîãè÷åñêèé èíñòèòóò Ðîññèéñêîé àêàäåìèè íàóê, Óíèâåðñèòåòñêàÿ íàá. 1, Ñàíêò-Ïåòåðáóðã 199034, Ðîññèÿ. KEY WORDS: Chrysomelidae, Eumolpinae, Medvedemolpus, new genus, new species. ÊËÞ×ÅÂÛÅ ÑËÎÂÀ: Chrysomelidae, Eumolpinae, Medvedemolpus, íîâûé ðîä, íîâûå âèäû. ABSTRACT. A new genus Medvedemolpus gen.n. Medevedemolpus gen.n. and to the genera of Philip- (tribe Nodinini; section Typophorites) and four new pinese Nodinini are given below. Some forms with species M. quadripunctatus, M. quinquepunctatus, unknown males continue to remain undescribed. M. basilianus and M. bakeri spp.n., are described from DEPOSITARIES: USNM National Museum of Philippines. Natural History, Smithsonian Institution, Washington; ZISP Zoological Institute of Russian Academy of ÐÅÇÞÌÅ. Ñ Ôèëèïïèíñêèõ îñòðîâîâ îïèñûâà- Sciences, St. Petersburg; cLM Lev Medvedevs col- åòñÿ íîâûé ðîä Medvedemolpus gen.n. (òðèáà Nodi- lection, Moscow. nini; ñåêöèÿ Typophorites) è ÷åòûðå íîâûõ âèäà M. Medvedemolpus gen.n. quadripunctatus, M. quinquepunctatus, M. basilianus è M. bakeri spp.n.. Type species M. bakeri Moseyko, sp.n. DESCRIPTION. Body glabrous, fulvous, partially with black spots, without metallic -

Multispecies Coalescent Analysis Unravels the Non-Monophyly and Controversial
bioRxiv preprint doi: https://doi.org/10.1101/187997; this version posted September 12, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Multispecies coalescent analysis unravels the non-monophyly and controversial relationships of Hexapoda Lucas A. Freitas, Beatriz Mello and Carlos G. Schrago* Departamento de Genética, Universidade Federal do Rio de Janeiro, RJ, Brazil *Address for correspondence: Carlos G. Schrago Universidade Federal do Rio de Janeiro Instituo de Biologia, Departamento de Genética, CCS, A2-092 Rua Prof. Rodolpho Paulo Rocco, S/N Cidade Universitária Rio de Janeiro, RJ CEP: 21.941-617 BRAZIL Phone: +55 21 2562-6397 Email: [email protected] Running title: Species tree estimation of Hexapoda Keywords: incomplete lineage sorting, effective population size, Insecta, phylogenomics bioRxiv preprint doi: https://doi.org/10.1101/187997; this version posted September 12, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract With the increase in the availability of genomic data, sequences from different loci are usually concatenated in a supermatrix for phylogenetic inference. However, as an alternative to the supermatrix approach, several implementations of the multispecies coalescent (MSC) have been increasingly used in phylogenomic analyses due to their advantages in accommodating gene tree topological heterogeneity by taking account population-level processes. Moreover, the development of faster algorithms under the MSC is enabling the analysis of thousands of loci/taxa. Here, we explored the MSC approach for a phylogenomic dataset of Insecta. -

Head Anatomy of Adult Nevrorthus Apatelios and Basal Splitting Events in Neuroptera (Neuroptera: Nevrorthidae)
72 (2): 111 – 136 27.7.2014 © Senckenberg Gesellschaft für Naturforschung, 2014. Head anatomy of adult Nevrorthus apatelios and basal splitting events in Neuroptera (Neuroptera: Nevrorthidae) Susanne Randolf *, 1, 2, Dominique Zimmermann 1, 2 & Ulrike Aspöck 1, 2 1 Natural History Museum Vienna, 2nd Zoological Department, Burgring 7, 1010 Vienna, Austria — 2 University of Vienna, Department of In- tegrative Zoology, Althanstrasse 14, 1090 Vienna, Austria; Susanne Randolf * [[email protected]]; Dominique Zimmermann [[email protected]]; Ulrike Aspöck [[email protected]] — * Corresponding author Accepted 22.v.2014. Published online at www.senckenberg.de/arthropod-systematics on 18.vii.2014. Abstract External and internal features of the head of adult Nevrorthus apatelios are described in detail. The results are compared with data from literature. The mouthpart muscle M. stipitalis transversalis and a hypopharyngeal transverse ligament are newly described for Neuroptera and herewith reported for the first time in Endopterygota. A submental gland with multiporous opening is described for Nevrorthidae and Osmylidae and is apparently unique among insects. The parsimony analysis indicates that Sisyridae is the sister group to all remaining Neuroptera. This placement is supported by the development of 1) a transverse division of the galea in two parts in all Neuroptera exclud ing Sisyridae, 2) the above mentioned submental gland in Nevrorthidae and Osmylidae, and 3) a poison system in all neuropteran larvae except Sisyridae. Implications for the phylogenetic relationships from the interpretation of larval character evolution, specifically the poison system, cryptonephry and formation of the head capsule are discussed. Key words Head anatomy, cladistic analysis, phylogeny, M. -

MEGALOPTERA: CORYDAL1DAE)L
Vol. 106, No.3, May &June, 1995 123 A REMARKABLE RANGE EXTENSION FOR THE FISHFLY GENUS DYSMICOHERMES (MEGALOPTERA: CORYDAL1DAE)l Atilano ~ontreras-~amos~ ABSTRACT: The megalopteran genus Dysmicohermes (Corydalidae: ~hauliodinae),previously known only from the Pacific Coast Region of the United States and adjacent Canada, is recorded for the first time in southeastern United States (Mission, Texas). The external genitalia of the sin- gle female Texan specimen most closely resemble those of D. ingens. However, differences in wing color pattern and body size, as well as the disjunct occurrence, suggest that the Texas spec- imen might belong to a third and new Dysmicohermes species. Survey work in southern Texas and adjadent Mexico is required in order to determine the taxonomic position, and to assess the con- servation status of this previously unknown fishfly. Fishflies of the genus Dysmicoherrnes (Corydalidae: Chauliodinae) are among the most impressive North American megalopterans. They have an average wing span of 120 mm (Evans 1972), which is comparable to a large Corydalus dobsonfly (Corydalidae: Corydalinae). Besides their large size, Dysmicohermes fishflies can be easily distinguished by having a 4-branched M vein in the hindwing (Evans and Neunzig 1984, New and Theischinger 1993) and by the presence of dense, long, curly hair on the thorax (Evans 1972). The two specimens I observed have hairs also on the head and coxae. Larvae of Dysmicohermes can be separated from other megalopteran genera with the keys by Evans and Neunzig (1984) and Neunzig and Baker (1991). Two species, Dysmicohermes disjunctus (Walker) and D. ingens Chandler, are presently included in the genus. -

Efficiency of Antlion Trap Construction
3510 The Journal of Experimental Biology 209, 3510-3515 Published by The Company of Biologists 2006 doi:10.1242/jeb.02401 Efficiency of antlion trap construction Arnold Fertin* and Jérôme Casas Université de Tours, IRBI UMR CNRS 6035, Parc Grandmont, 37200 Tours, France *Author for correspondence (e-mail: [email protected]) Accepted 21 June 2006 Summary Assessing the architectural optimality of animal physical constant of sand that defines the steepest possible constructions is in most cases extremely difficult, but is slope. Antlions produce efficient traps, with slopes steep feasible for antlion larvae, which dig simple pits in sand to enough to guide preys to their mouths without any attack, catch ants. Slope angle, conicity and the distance between and shallow enough to avoid the likelihood of avalanches the head and the trap bottom, known as off-centring, were typical of crater angles. The reasons for the paucity of measured using a precise scanning device. Complete attack simplest and most efficient traps such as theses in the sequences in the same pits were then quantified, with animal kingdom are discussed. predation cost related to the number of behavioural items before capture. Off-centring leads to a loss of architectural efficiency that is compensated by complex attack Supplementary material available online at behaviour. Off-centring happened in half of the cases and http://jeb.biologists.org/cgi/content/full/209/18/3510/DC1 corresponded to post-construction movements. In the absence of off-centring, the trap is perfectly conical and Key words: animal construction, antlion pit, sit-and-wait predation, the angle is significantly smaller than the crater angle, a physics of sand, psammophily. -

Final Report 1
Sand pit for Biodiversity at Cep II quarry Researcher: Klára Řehounková Research group: Petr Bogusch, David Boukal, Milan Boukal, Lukáš Čížek, František Grycz, Petr Hesoun, Kamila Lencová, Anna Lepšová, Jan Máca, Pavel Marhoul, Klára Řehounková, Jiří Řehounek, Lenka Schmidtmayerová, Robert Tropek Březen – září 2012 Abstract We compared the effect of restoration status (technical reclamation, spontaneous succession, disturbed succession) on the communities of vascular plants and assemblages of arthropods in CEP II sand pit (T řebo ňsko region, SW part of the Czech Republic) to evaluate their biodiversity and conservation potential. We also studied the experimental restoration of psammophytic grasslands to compare the impact of two near-natural restoration methods (spontaneous and assisted succession) to establishment of target species. The sand pit comprises stages of 2 to 30 years since site abandonment with moisture gradient from wet to dry habitats. In all studied groups, i.e. vascular pants and arthropods, open spontaneously revegetated sites continuously disturbed by intensive recreation activities hosted the largest proportion of target and endangered species which occurred less in the more closed spontaneously revegetated sites and which were nearly absent in technically reclaimed sites. Out results provide clear evidence that the mosaics of spontaneously established forests habitats and open sand habitats are the most valuable stands from the conservation point of view. It has been documented that no expensive technical reclamations are needed to restore post-mining sites which can serve as secondary habitats for many endangered and declining species. The experimental restoration of rare and endangered plant communities seems to be efficient and promising method for a future large-scale restoration projects in abandoned sand pits. -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

André Nel Sixtieth Anniversary Festschrift
Palaeoentomology 002 (6): 534–555 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY PE Copyright © 2019 Magnolia Press Editorial ISSN 2624-2834 (online edition) https://doi.org/10.11646/palaeoentomology.2.6.1 http://zoobank.org/urn:lsid:zoobank.org:pub:25D35BD3-0C86-4BD6-B350-C98CA499A9B4 André Nel sixtieth anniversary Festschrift DANY AZAR1, 2, ROMAIN GARROUSTE3 & ANTONIO ARILLO4 1Lebanese University, Faculty of Sciences II, Department of Natural Sciences, P.O. Box: 26110217, Fanar, Matn, Lebanon. Email: [email protected] 2State Key Laboratory of Palaeobiology and Stratigraphy, Center for Excellence in Life and Paleoenvironment, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, China. 3Institut de Systématique, Évolution, Biodiversité, ISYEB-UMR 7205-CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 57 rue Cuvier, CP 50, Entomologie, F-75005, Paris, France. 4Departamento de Biodiversidad, Ecología y Evolución, Facultad de Biología, Universidad Complutense, Madrid, Spain. FIGURE 1. Portrait of André Nel. During the last “International Congress on Fossil Insects, mainly by our esteemed Russian colleagues, and where Arthropods and Amber” held this year in the Dominican several of our members in the IPS contributed in edited volumes honoring some of our great scientists. Republic, we unanimously agreed—in the International This issue is a Festschrift to celebrate the 60th Palaeoentomological Society (IPS)—to honor our great birthday of Professor André Nel (from the ‘Muséum colleagues who have given us and the science (and still) national d’Histoire naturelle’, Paris) and constitutes significant knowledge on the evolution of fossil insects a tribute to him for his great ongoing, prolific and his and terrestrial arthropods over the years. -

Bulletin Number / Numéro 2 Entomological Society of Canada Société D’Entomologie Du Canada June / Juin 2008
Volume 40 Bulletin Number / numéro 2 Entomological Society of Canada Société d’entomologie du Canada June / juin 2008 Published quarterly by the Entomological Society of Canada Publication trimestrielle par la Société d’entomologie du Canada ............................................................... .................................................................................................................................................................................................................................................................................................................................. .......................................................................... ........................................................................................................................................................................ ....................... ................................................................................. ................................................. List of contents / Table des matières Volume 40 (2), June / june 2008 Up front / Avant-propos ................................................................................................................49 Moth balls / Boules à mites .............................................................................................................51 Meeting announcements / Réunions futures ..................................................................................52 Dear Buggy / Cher Bibitte ..............................................................................................................53 -
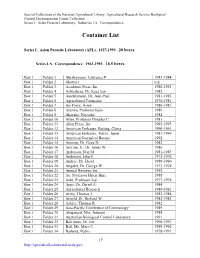
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

Folk Taxonomy, Nomenclature, Medicinal and Other Uses, Folklore, and Nature Conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3
Ulicsni et al. Journal of Ethnobiology and Ethnomedicine (2016) 12:47 DOI 10.1186/s13002-016-0118-7 RESEARCH Open Access Folk knowledge of invertebrates in Central Europe - folk taxonomy, nomenclature, medicinal and other uses, folklore, and nature conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3 Abstract Background: There is scarce information about European folk knowledge of wild invertebrate fauna. We have documented such folk knowledge in three regions, in Romania, Slovakia and Croatia. We provide a list of folk taxa, and discuss folk biological classification and nomenclature, salient features, uses, related proverbs and sayings, and conservation. Methods: We collected data among Hungarian-speaking people practising small-scale, traditional agriculture. We studied “all” invertebrate species (species groups) potentially occurring in the vicinity of the settlements. We used photos, held semi-structured interviews, and conducted picture sorting. Results: We documented 208 invertebrate folk taxa. Many species were known which have, to our knowledge, no economic significance. 36 % of the species were known to at least half of the informants. Knowledge reliability was high, although informants were sometimes prone to exaggeration. 93 % of folk taxa had their own individual names, and 90 % of the taxa were embedded in the folk taxonomy. Twenty four species were of direct use to humans (4 medicinal, 5 consumed, 11 as bait, 2 as playthings). Completely new was the discovery that the honey stomachs of black-coloured carpenter bees (Xylocopa violacea, X. valga)were consumed. 30 taxa were associated with a proverb or used for weather forecasting, or predicting harvests. Conscious ideas about conserving invertebrates only occurred with a few taxa, but informants would generally refrain from harming firebugs (Pyrrhocoris apterus), field crickets (Gryllus campestris) and most butterflies.