Technical Articles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
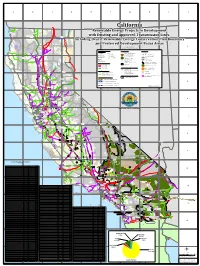
California Renewable Energy Projects in Development with Existing And
A B C D E F G H I J California ¨¦§5 2 ¤£101 !( Crescent Renewable Energy Projects in Development City Yreka D e l N o r t e !( C o u n t y S i s k i y o u M o d o c with Existing and Approved Transmission Lines C o u n t y C o u n t y Alturas !( Including Desert Renewable Energy Conservation Plan Boundary ¤£395 and Preferred Development Focus Areas Updated 12/17/2015 3 Prepared by the California Energy Commission S h a s t a H u m b o l d t C o u n t y Renewable Energy Projects Eureka C o u n t y Land Ownerships Other Features !( L a s s e n In Development C o u n t y Biomass/ Solar PV Bureau of Land Management (! City Weaverville (! !( [[ !( Landfill Gas 1 - 20 MW )" [ [ Existing Substation* [ Department of Defense 5 (! Geothermal (! )" ¨¦§ 21 - 200 MW Bureau of Indian Affairs Approved Substation* 101 Redding !( ¤£ T r i n i t y !( (! Small Hydro C o u n t y (! 201 - 2,400 MW California State Park Road (! Wind State Owned Land County Boundary Susanville !( (! Solar Thermal Williamson Act NOTE: Williamson Act for Alpine, Del Norte, Inyo, Mono, Orange, San Benito, San Francisco and Yuba Counties not available Desert Renewable Energy Existing Transmission Line Conservation Plan Boundary Private Land 4 Red 345 - 500kV Bluff DRECP Conservation Lands !( 200 - 287kV Te h a m a Prohibited, Restricted & Limited Lands C o u n t y 110 - 161kV Mohave Ground Squirrel ¤£395 Category I Lands Key Population Center Energy Development Prohibited or Restricted by Policy Quincy !( Federal Energy Corridors P l u m a s Category II Lands Dry Lake C o u n t -

The Economics of Solar Power
The Economics of Solar Power Solar Roundtable Kansas Corporation Commission March 3, 2009 Peter Lorenz President Quanta Renewable Energy Services SOLAR POWER - BREAKTHROUGH OR NICHE OPPORTUNITY? MW capacity additions per year CAGR +82% 2000-08 Percent 5,600-6,000 40 RoW US 40 +43% Japan 10 +35% 2,826 Spain 55 1,744 1,460 1,086 598 Germany 137 241 372 427 2000 01 02 03 04 05 06 07 2008E Demand driven by attractive economics • Strong regulatory support • Increasing power prices • Decreasing solar system prices • Good availability of capital Source: McKinsey demand model; Solarbuzz 1 WE HAVE SEEN SOME INTERESTING CHANGES IN THE U.S. RECENTLY 2 TODAY’S DISCUSSION • Solar technologies and their evolution • Demand growth outlook • Perspectives on solar following the economic crisis 3 TWO KEY SOLAR TECHNOLOGIES EXIST Photovoltaics (PV) Concentrated Solar Power (CSP) Key • Uses light-absorbing material to • Uses mirrors to generate steam characteristics generate current which powers turbine • High modularity (1 kW - 50 MW) • Low modularity (20 - 300 MW) • Uses direct and indirect sunlight – • Only uses direct sunlight – specific suitable for almost all locations site requirements • Incentives widely available • Incentives limited to few countries • Mainly used as distributed power, • Central power only limited by some incentives encourage large adequate locations and solar farms transmission access ~ 10 Global capacity ~ 0.5 GW, 2007 Source: McKinsey analysis; EPIA; MarketBuzz 4 THESE HAVE SEVERAL SUB-TECHNOLOGIES Key technologies Sub technologiesDescription -

Appendix O, Existing Renewable Energy Projects Within DRECP
Appendix O Existing Renewable Energy Projects Within DRECP Plan Area Draft DRECP and EIR/EIS APPENDIX O. EXISTING RENEWABLE ENERGY PROJECTS WITHIN PLAN AREA O EXISTING RENEWABLE ENERGY PROJECTS WITHIN PLAN AREA Background. The list of projects included in this file was developed to help define the number and capacity of existing renewable energy projects in the Plan Area. These projects are considered part of the affected environment in the EIR/EIS Volume III. A map indicating the locations of these projects is presented as Figure III.1-2. Existing projects include those that are either operational or under construction as of October 2013. Two tables are attached: Table O-1: Summary of Existing RE Projects (data summarized from Table O-2) Table O-2: Existing RE Projects (all projects) Table O-1 below presents the acreage of existing renewable energy projects in the DRECP Plan Area. For informational purposes, 57% (50,337 acres) of the land affected by existing renewable energy projects are located in the Preferred Alternative DFAs, as defined in Volume II. This represents less than 2.5% of the DFA acreage for the Preferred Alternative. Table O-1 Summary of Existing Renewable Energy (RE) Projects in the DRECP Plan Area Generation Capacity Acreage Percent of Percent Total Acres Total Total of Percent of RE Projects Acres of RE Project Total Total of Total in the RE Projects Acreage MW MW Acres Plan Area in DFAs* in DFAs* BLM-Administered 1,805 29% 28% 24,593 8,686 35% Public Lands – Total Wind 615 34% 61% 15,028 2,592 11% Solar 1,190 66% 39% 9,565 6,094 25% Private Lands and 4,445 71% 72% 64,463 41,634 65% Other Public Lands – Total Wind 2,015 45% 69% 44,643 23,222 36% Solar 2,330 53% 31% 19,265 18,419 29% Geothermal 100 2% 0.1% 545 545 0.1% Total RE Projects 6,250 100% 100% 89,046 50,337 57% *The DFA locations for the Preferred Alternative are used to define in this exercise. -

BC-TES-TMP-2443.Pdf (5.514Mb)
UNIVERSIDAD NACIONAL “PEDRO RUIZ GALLO” Facultad de Ingeniería Mecánica y Eléctrica TESIS Para Optar el Título Profesional de INGENIERO MECÁNICO ELECTRICISTA “UTILIZACIÓN DE LA ENERGÍA EÓLICA Y SOLAR COMO FUENTE PARA EL SUMINISTRO DE ENERGÍA ELÉCTRICA AL CASERÍO ALTO PONGOYA EN CHIMBAN PROVINCIA DE CHOTA DEPARTAMENTO DE CAJAMARCA” Presentado Por: Bach. KEVIN ARNOLD TARRILLO VÁSQUEZ Asesor: Msc. Ing. JONY VILLALOBOS CABRERA LAMBAYEQUE – PERÚ Enero del 2019 UNIVERSIDAD NACIONAL “PEDRO RUIZ GALLO” Facultad de Ingeniería Mecánica y Eléctrica TESIS Para Optar el Título Profesional de INGENIERO MECÁNICO ELECTRICISTA “UTILIZACIÓN DE LA ENERGÍA EÓLICA Y SOLAR COMO FUENTE PARA EL SUMINISTRO DE ENERGÍA ELÉCTRICA ALELECTRICISTA CASERÍO ALTO PONGOYA EN CHIMBAN PROVINCIA DE CHOTA DEPARTAMENTO DE CAJAMARCA” Presentado Por: Bach. KEVIN ARNOLD TARRILLO VÁSQUEZ Aprobado por el Jurado Examinador PRESIDENTE: Dr. DANIEL CARRANZA MONTENEGRO. SECRETARIO: ING. CARLOS JAVIER COTRINA SAAVEDRA. MIEMBRO: ING. TEOBALDO EDGAR JULCA OROZCO. ASESOR: M.Sc. JONY VILLALOBOS CABRERA. LAMBAYEQUE – PERÚ Enero del 2019 UNIVERSIDAD NACIONAL “PEDRO RUIZ GALLO” Facultad de Ingeniería Mecánica y Eléctrica TESIS TITULO “UTILIZACIÓN DE LA ENERGÍA EÓLICA Y SOLAR COMO FUENTE PARA EL SUMINISTRO DE ENERGÍA ELÉCTRICA AL CASERÍO ALTO PONGOYA EN CHIMBAN PROVINCIA DE CHOTA DEPARTAMENTO DE CAJAMARCA” CONTENIDOS CAPITULO I: PROBLEMA DE LA INVESTIGACIÓN. CAPITULO II: MARCO TEÓRICO. CAPITULO III: MARCO METODOLÓGICO. CAPITULO IV: PROPUESTA DE LA INVESTIGACIÓN. CAPITULO V: ANÁLISIS E INTERPRETACIÓN DE LOS RESULTADOS. CAPITULO VI: CONCLUSIONES Y RECOMENDACIONES. AUTOR: Bach. KEVIN ARNOLD TARRILLO VÁSQUEZ --------------------------------- --------------------------------------- PRESIDENTE SECRETARIO ---------------------------------- ------------------------------------- MIEMBRO ASESOR LAMBAYEQUE – PERÚ Enero del 2019 DEDICATORIA Dedico éste Proyecto de Tesis a: Dios ya que gracias a Él he llegado a concluir una de mis metas la cual es concluir con mi carrera. -

Solar Power Card U.S
NORTH SCORE AMERICAN SOLAR POWER CARD U.S. SOLAR POWER Canada Solar Power Total grid-connected PV generating capacity for the U.S., as of the Total PV grid-connected capacity, end of 2019: 3,196 MW end of Q1, 2020: 81,400 megawatts (MW) Installed in 2019: 102 MW Growth in PV generated capacity during 2019: 13,300 MW of new solar PV ✷ Solar power accounted for nearly 40 percent of all new electricity generating capacity added in the U.S. in 2019, the largest annual share in the industry’s history. Canadian Solar Power Initiatives ✷ The U.S. solar market installed 3.6 gigawatts (GW) of new solar photovoltaic (PV) capacity in Q1 2020, representing its largest first quarter ever in the U.S. ✷ The Government of Canada launched the long-awaited Greening Government initiative, a power purchase agreement (PPA) program, with a request for information regarding The COVID-19 pandemic is having a significant impact on the U.S. solar industry, but overall, the ✷ the procurement of up to 280,000 MWh per year in newly-built solar PV and wind generation Solar Energy Industries Association (SEIA) and consulting firm Wood Mackenzie forecast 33 percent capacity. It is designed to offset federal government operations within the province of growth in 2020, owing entirely to the strong performance of the utility-scale segment, which is Alberta, as well as an additional 240,000 – 360,000 MWh per year in Renewable Energy expected to account for more than 14 GW of new installations this year. Certificates (REC) to offset Federal electricity emissions in other provinces. -

Financing the Transition to Renewable Energy in the European Union
Bi-regional economic perspectives EU-LAC Foundation Miguel Vazquez, Michelle Hallack, Gustavo Andreão, Alberto Tomelin, Felipe Botelho, Yannick Perez and Matteo di Castelnuovo. iale Luigi Bocconi Financing the transition to renewable energy in the European Union, Latin America and the Caribbean Financing the transition to renewable energy in European Union, Latin America and Caribbean EU-LAC / Università Commerc EU-LAC FOUNDATION, AUGUST 2018 Große Bleichen 35 20354 Hamburg, Germany www.eulacfoundation.org EDITION: EU-LAC Foundation AUTHORS: Miguel Vazquez, Michelle Hallack, Gustavo Andreão, Alberto Tomelin, Felipe Botelho, Yannick Perez and Matteo di Castelnuovo GRAPHIC DESIGN: Virginia Scardino | https://www.behance.net/virginiascardino PRINT: Scharlau GmbH DOI: 10.12858/0818EN Note: This study was financed by the EU-LAC Foundation. The EU-LAC Foundation is funded by its members, and in particular by the European Union. The contents of this publication are the sole responsibility of the authors and cannot be considered as the point of view of the EU- LAC Foundation, its member states or the European Union. This book was published in 2018. This publication has a copyright, but the text may be used free of charge for the purposes of advocacy, campaigning, education, and research, provided that the source is properly acknowledged. The co- pyright holder requests that all such use be registered with them for impact assessment purposes. For copying in any other circumstances, or for reuse in other publications, or for translation and adaptation, -

Integrating Iot Devices and Deep Learning for Renewable Energy in Big Data System
U.P.B. Sci. Bull., Series C , Vol. 82, Iss. 3, 2020 ISSN 2286-3540 INTEGRATING IOT DEVICES AND DEEP LEARNING FOR RENEWABLE ENERGY IN BIG DATA SYSTEM Med Anouar NAOUI1, Brahim LEJDEL2, Mouloud AYAD3 Renewable energy use has increased rapidly in recent years. As a result, it has attracted many researchers and industries to provide safe and sustainable energy to customers. However, the high use of renewable energy has caused many problems in its forecasts. To provide energy forecasts, data was collected from wind turbines or solar panels. Several approaches have been proposed for renewable energy forecasting, but they do not take into account Big data characteristics. These approaches focus only on data analysis and are limited. This paper proposes an ideal architecture that integrates IoT technology, Big Data system and Deep Learning paradigm for energy forecasting. The proposed architecture is divided into four layers: renewable energy layer, Big Data layer, IoT layer and Deep Learning layer. Renewable energy data are Big, thus a Big data layer is proposed for the storage and processing of renewable energy data. Then, the Deep Learning layer is used to predict renewable energy. A distributed LSTM algorithm is implemented and compared with three different models to illustrate the predictability and optimization of execution time in the proposed system. Finally, IoT technology is used to facilitate the acquisition of weather, wind or solar data. Keywords: Deep learning, Big data, Internet of things, Distributed long short term memory. 1. Introduction The use of renewable energy resources is increasing rapidly worldwide. In 2017, renewable energy consummation represented up to 17% of total global energy consumption. -

CLIMATE CHANGE BUSINESS JOURNAL® Volume V, No
on for the Clim mati ate C for han In g ic e I g nd te u a s r t t r S y CLIMATE CHANGE BUSINESS JOURNAL® Volume V, No. 11/12 November/December 2012 Climate Change Business Journal 2012 Business 2012 Executive Review & CCBJ Awards Achievement Awards CCBJ’s 2012 Business Achievement Awards recognize more than 30 high achievers in the climate change industry. Senior executives and top experts in the Business Achievement: Growth industry share their perspectives in Q&A sessions and bylined guest articles. Gold Q&As & Articles Inside this Issue: rightSource Energy (Oakland, 11: Top 12 CCBJ quotes from 2012 30: Greenhouse Gas Management Institute focuses on professional development and Calif.) for becoming the clear 12: Then & Now: Skeptics who changed education. leader in the U.S. concentrat- their minds about climate change ing solar power (CSP) segment. At a 34: Powerit offers automated solutions to B 18: AECOM on risk management and time when most CSP projects proposed industrial demand response market. over the last four years in the United climate change adaptation. 37: WSP finds mitigation and adaptation States have been delayed, canceled or 21: Jane Capital offers perspective on smart figuring large in the in supply chain. reconfigured as solar PV projects, Bright- grid investments. Source has pushed ahead with its 377 40: WESTON describes how sustainability 24: Pike Research addresses distributed MW Ivanpah Solar Energy Generating unites a diverse practice. generation and microgrids. System (86% owned by NRG Energy 43: CH2M HILL engages with climate risk and Google) in the southwest California 26: Real estate services firm CBRE looks at worldwide. -

Helios, Silfab E Le Macerie Del Governo
ANNO 4 - N°45 - 10 DICEMBRE 2012 - LA NEWSLETTER PER GLI OPERATORI DI SETTORE Leader Europei nell’integrazione architettonica. Moduli speciali CAPILLARY COMB made in EU. www.sunage.ch CONTO ENERGIA EDITORIALE AZIENDE E BRAND Impianti in esercizio: 461.981 CITATI IN QUESTO NUMERO Potenza: 16.108,5 MW HELIOS, SILFAB Aeeg Afase Costo indicativo annuo E LE MACERIE DEL GOVERNO AFHolding degli incentivi: Aión Renewables 6.490,9 milioni di euro Fa una certa impressione assistere al tramonto di un Governo proprio nel momento in cui vengono a galla le drammatiche conseguenze delle deli- Anie/Gifi Alla fine del 5° Conto berate scelte di uno dei suo dicasteri più importanti: quello dello Sviluppo Anie Energia Energia mancano: Aros 209,1 milioni di euro Economico. Assosolare Mentre il tema della mancanza di una strategia industriale per il Paese AvProject Installati da inizio 2012: riempie i dibattiti in Tv e sui giornali, anche sull’onda delle vicende dell’Il- Bester Generación 3.647,5 MW Bisol Group Installati 5° Conto Energia: va e della Fiat, l’industria italiana del fotovoltaico continua a perdere pezzi. 413,9 MW Centrosolar Nei giorni scorsi Aión Renewables ha depositato la domanda di con- Cobat Potenza media 5° Conto cordato preventivo per Helios Technology. E da Padova arrivano notizie Coenergia Energia: 26,4 kWp Confindustria Ancona segue a pagina 2 Potenza media da inizio Confindustria Energia 2012: 25,4 kWp Danfoss Potenza media totale Elettronica Santerno installato: 34,9 KWp News dal 3 al 7 dicembre 2012 Enecsys NB: Non sono compresi gli Energy Resources impianti iscritti in posizione Dal 18 al 20 aprile tornano a Fabriano EnergyLink utile al 1° registro del 5° General Membrane Conto Energia e non ancora “Le giornate dell’energia” Gruppo Carraro in esercizio 07/12. -

Q-Cells Australia
ANNUAL REPORT 2010 PROGRESS ANNUAL REPORT 2010 CONTENTS MARKET SEGMENTS BRANDED OEM PRIVATE ROOFTOP ARRAYS LARGE SOLAR (CELLS) ROOFTOPS AND ON SMALL POWER PLANTS IN-ROOF-ARRAYS COMMERCIAL AND AND SYSTEMS INDUSTRIAL BUILDINGS Module Distributors, Large C & I, Utility, TARGET GROUPS manufacturers large installers segment integrators customers / IPP’s PRODUCTS Solar cells Mono Multi PROGRESS 4 NEWSolar PRODUCTS modules Q.PEAK * 12 NEW MARKETS Q.SMART 24 NEW DEVELOPMENTSQ.PRO Q.BASE MANAGEMENTSolar systems GROUP MANAGEMENT REPORT CONSOLIDATED 32 INTERVIEW WITH THE CEO 66 BUSINESS AND FINANCIAL STATEMENTS 35 MEMBERS Product utilisation OF THE per BOARD segment. OPERATING ENVIRONMENT 110 CONSOLIDATED * Introduction planned for the second half-year 2011. INCOME STATEMENT 36 REPORT OF THE 76 INCOME, FINANCIAL AND SUPERVISORY BOARD NET ASSET POSITION 111 CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME 41 CORPORATE GOVERNANCE 87 OTHER DISCLOSURES 112 CONSOLIDATED BALANCE SHEET 50 CORPORATE RESPONSIBILITY 91 RISK REPORT 114 CONSOLIDATED 60 Q-CELLS AT THE CAPITAL MARKET 102 SUPPLEMENTAL REPORT CASH FLOW STATEMENT 103 FORECAST REPORT 116 CHANGES IN CONSOLIDATED EQUITY 118 NOTES TO THE CONSOLIDATED 2010 RESULTS FINANCIAL STATEMENTS 192 LIST OF SHAREHOLDINGS FURTHER INFORMATION Target What we achieved in 2010 196 AUDITOR’S REPORT Building up our innovative • We have added to our product portfolio and offer solutions for 197 all applications. RESPONSIBILITY STATEMENT product portfolio for all • We have successfully launched our crystalline Q.PRO and Q.BASE solarOF THE modules COMPANY’S on the market. photovoltaic applications • Our Q.SMART thin-film modules have achieved a record-breaking levelLEGAL of efficiency. REPRESENTATIVES • We have upped our production volume significantly: 2010 saw the production of solar cells and modules with a total volume of over 1 GWp for the first time. -

Little Room Left for Improvement the SELF-CONSUMPTION SMART INVERTER
THE MAGAZINE for Renewable Energies ISSN 1861-2741 74714 www.sunwindenergy.com € 12.00 • International issue HEATING WITH PV Making good use of surpluses BLOCKCHAIN The new buzzword of the energy market 5/2016 TheTHE solar SOLAR edi EDITIONTion MARKET OVERVIEW COLLECTORS Little room left for improvement THE SELF-CONSUMPTION SMART INVERTER • ON-GRID, OFF-GRID, BACK-UP ALL IN ONE • SMART STORAGE MANAGEMENT • PLUG-&-PLAY INSTALLATION Smart Grid Inverter • LO C A L & R E M O T E Produce and Use Your Own Energy ! MONITORING IMEON 3.6 IMEON 9.12 Up to 4kWp Monophase Up to 12kWp Triphase All the Smart Grid in one inverter Find out more, or contact your IMEON Smart Grid inverter technology is the all-in-one answer for true multi-energy sources nearest IMEON distributor management. Consuming one’s own solar production directly, storing in batteries for later www.imeon-energy.com use or in case of power cuts, and also injecting to - or consuming from - the grid only when needed, is now all possible. Cutting-edge French research and innovation helped develop this IMEON ENERGY built-in intelligence and energy management to finally enable real control over one’s power. 10 Rue Amiral Romain Desfossés 29200 Brest - FRANCE +33 1 84 17 51 15 NRS [email protected] IMEON Full Page ad_Sun&Wind-EN- 31-08-2016.indd 1 31/08/2016 09:25:16 EDITORIAL IEA acknowledges the global change in energy systems ooking back, this decade may be seen then there was a growth there of over a third, Dr. -

Operation Construction Development
Major Solar Projects in the United States Operating, Under Construction, or Under Development Updated March 7, 2016 Overview This list is for informational purposes only, reflecting projects and completed milestones in the public domain. The information in this list was gathered from public announcements of solar projects in the form of company press releases, news releases, and, in some cases, conversations with individual developers. It is not a comprehensive list of all major solar projects under development. This list may be missing smaller projects that are not publicly announced. Particularly, many smaller projects located outside of California that are built on a short time-scale may be underrepresented on this list. Also, SEIA does not guarantee that every identified project will be built. Like any other industry, market conditions may impact project economics and timelines. SEIA will remove a project if it is publicly announced that it has been cancelled. SEIA actively promotes public policy that minimizes regulatory uncertainty and encourages the accelerated deployment of utility-scale solar power. This list includes ground-mounted solar power plants 1 MW and larger. Example Projects Nevada Solar One Sierra SunTower Nellis Air Force Base DeSoto Next Generation Solar Energy Center Developer: Acciona Developer: eSolar Developer: MMA Renewable Ventures Developer: Florida Power & Light Co. Electricity Purchaser: NV Energy Electricity Purchaser: Southern Electricity Purchaser: Nellis AFB Electricity Purchaser: Florida Power & California