Ventegra Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stanford Chem-H Presentation (PDF)
KiNativ® In situ kinase profiling Stanford University ChEM-H confidential @KiNativPlatform Principle of the KiNativ platform • ATP (or ADP) acyl phosphate binds to, and covalently modifies Lysine residues in the active site • Thus, ATP acyl phosphate with a desthiobiotin tag can be used capture and quantitate kinases in a complex lysate Acyl phosphate Desthiobiotin tag ATP 2 ATP acyl phosphate probe covalently modifies kinase in the active site Lysine 2 Lysine 1 3 ATP acyl phosphate probe covalently modifies kinase in the active site Lysine 2 Lysine 1 4 Samples trypsinized, probe-labeled peptides captured with streptavidin, and analyzed by targeted LC-MS2 Identification Quantitation Explicit determination of peptide Integration of signal from MS2 sequence and probe modification site fragment ions from MS2 spectrum 5 Comprehensive Coverage of Protein and Lipid Kinases Protein kinases Atypical kinases Green: Kinases detected on KiNativ Red: Kinases not detected on KiNativ ~80% of known protein and atypical kinases identified on the platform http://www.kinativ.com/coverage/protein-lipid.html 6 Profiling compound(s) on the KiNativ platform Control sample – add probe Sample: Lysate derived from any cell line or tissue from ANY species Treated sample – add inhibitor followed by probe Inhibited kinase Green: Kinases Blue: Probe Gray: Non-kinases Red: Inhibitor 7 Profiling compound(s) on the KiNativ platform Control sample – add probe MS signalMS Sample: Lysate derived from any cell line or tissue from ANY species Treated sample – add inhibitor -

Refractory Early T-Cell Precursor Acute Lymphoblastic Leukemia
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Venetoclax and Bortezomib in Relapsed/Refractory Early T-Cell Precursor Acute Lymphoblastic Leukemia La Starza, Roberta ; Cambò, Benedetta ; Pierini, Antonio ; Bornhauser, Beat ; Montanaro, Anna ; Bourquin, Jean-Pierre ; Mecucci, Cristina ; Roti, Giovanni DOI: https://doi.org/10.1200/PO.19.00172 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-198023 Journal Article Published Version The following work is licensed under a Creative Commons: Attribution 4.0 International (CC BY 4.0) License. Originally published at: La Starza, Roberta; Cambò, Benedetta; Pierini, Antonio; Bornhauser, Beat; Montanaro, Anna; Bourquin, Jean-Pierre; Mecucci, Cristina; Roti, Giovanni (2019). Venetoclax and Bortezomib in Relapsed/Refrac- tory Early T-Cell Precursor Acute Lymphoblastic Leukemia. JCO precision oncology, 3:PO.19.00172. DOI: https://doi.org/10.1200/PO.19.00172 case report Venetoclax and Bortezomib in Relapsed/ Refractory Early T-Cell Precursor Acute Lymphoblastic Leukemia Roberta La Starza, MD, PhD1; Benedetta Cambo,` MD2; Antonio Pierini, MD, PhD1; Beat Bornhauser, PhD3; Anna Montanaro2; Jean-Pierre Bourquin, MD, PhD3; Cristina Mecucci, MD, PhD1; and Giovanni Roti, MD, PhD2 INTRODUCTION CD2, CD4, CD8, and CD1a and positive for human Although in the past three decades we welcomed the leukocyte antigen (HLA-DR), CD38, CD117, CD33, advent of targeted therapies in -

A Phase I Study of Pexidartinib, a Colony-Stimulating Factor 1 Receptor Inhibitor, in Asian Patients with Advanced Solid Tumors
Investigational New Drugs https://doi.org/10.1007/s10637-019-00745-z PHASE I STUDIES A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in Asian patients with advanced solid tumors Jih-Hsiang Lee1 & Tom Wei-Wu Chen2 & Chih-Hung Hsu2,3 & Yu-Hsin Yen2 & James Chih-Hsin Yang2,3 & Ann-Lii Cheng2,3 & Shun-ichi Sasaki4 & LiYin (Lillian) Chiu5 & Masahiro Sugihara4 & Tomoko Ishizuka4 & Toshihiro Oguma4 & Naoyuki Tajima4 & Chia-Chi Lin2,6 Received: 15 January 2019 /Accepted: 7 February 2019 # The Author(s) 2019 Summary Background Pexidartinib, a novel, orally administered small-molecule tyrosine kinase inhibitor, has strong selectivity against colony- stimulating factor 1 receptor. This phase I, nonrandomized, open-label multiple-dose study evaluated pexidartinib safety and efficacy in Asian patients with symptomatic, advanced solid tumors. Materials and Methods Patients received pexidartinib: cohort 1, 600 mg/d; cohort 2, 1000 mg/d for 2 weeks, then 800 mg/d. Primary objectives assessed pexidartinib safety and tolerability, and determined the recommended phase 2 dose; secondary objectives evaluated efficacy and pharmacokinetic profile. Results All11patients(6males,5 females; median age 64, range 23–82; cohort 1 n = 3; cohort 2 n = 8) experienced at least one treatment-emergent adverse event; 5 experienced at least one grade ≥ 3 adverse event, most commonly (18%) for each of the following: increased aspartate aminotransfer- ase, blood alkaline phosphatase, gamma-glutamyl transferase, and anemia. Recommended phase 2 dose was 1000 mg/d for 2 weeks and800mg/dthereafter.Pexidartinibexposure,areaundertheplasmaconcentration-timecurvefromzeroto8h(AUC0-8h), and maximum observed plasma concentration (Cmax) increased on days 1 and 15 with increasing pexidartinib doses, and time at Cmax (Tmax) was consistent throughout all doses. -

Press Release
Press Release Daiichi Sankyo and AstraZeneca Announce Global Development and Commercialization Collaboration for Daiichi Sankyo’s HER2 Targeting Antibody Drug Conjugate [Fam-] Trastuzumab Deruxtecan (DS-8201) Collaboration combines Daiichi Sankyo’s scientific and technological excellence with AstraZeneca’s global experience and resources in oncology to accelerate and expand the potential of [fam-] trastuzumab deruxtecan as monotherapy and combination therapy across a spectrum of HER2 expressing cancers AstraZeneca to pay Daiichi Sankyo up to $6.90 billion in total consideration, including $1.35 billion upfront payment and up to an additional $5.55 billion contingent upon achievement of future regulatory and sales milestones as well as other contingencies Companies to share equally development and commercialization costs as well as profits worldwide from [fam-] trastuzumab deruxtecan with Daiichi Sankyo maintaining exclusive rights in Japan Daiichi Sankyo is expected to book sales in U.S., certain countries in Europe, and certain other markets where Daiichi Sankyo has affiliates; AstraZeneca is expected to book sales in all other markets worldwide, including China, Australia, Canada and Russia Tokyo, Munich and Basking Ridge, NJ – (March 28, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) announced today that it has entered into a global development and commercialization agreement with AstraZeneca for Daiichi Sankyo’s lead antibody drug conjugate (ADC), [fam-] trastuzumab deruxtecan (DS-8201), currently in pivotal development for multiple HER2 expressing cancers including breast and gastric cancer, and additional development in non-small cell lung and colorectal cancer. Daiichi Sankyo and AstraZeneca will jointly develop and commercialize [fam-] trastuzumab deruxtecan as a monotherapy or a combination therapy worldwide, except in Japan where Daiichi Sankyo will maintain exclusive rights. -

Identification of Candidate Repurposable Drugs to Combat COVID-19 Using a Signature-Based Approach
www.nature.com/scientificreports OPEN Identifcation of candidate repurposable drugs to combat COVID‑19 using a signature‑based approach Sinead M. O’Donovan1,10, Ali Imami1,10, Hunter Eby1, Nicholas D. Henkel1, Justin Fortune Creeden1, Sophie Asah1, Xiaolu Zhang1, Xiaojun Wu1, Rawan Alnafsah1, R. Travis Taylor2, James Reigle3,4, Alexander Thorman6, Behrouz Shamsaei4, Jarek Meller4,5,6,7,8 & Robert E. McCullumsmith1,9* The COVID‑19 pandemic caused by the novel SARS‑CoV‑2 is more contagious than other coronaviruses and has higher rates of mortality than infuenza. Identifcation of efective therapeutics is a crucial tool to treat those infected with SARS‑CoV‑2 and limit the spread of this novel disease globally. We deployed a bioinformatics workfow to identify candidate drugs for the treatment of COVID‑19. Using an “omics” repository, the Library of Integrated Network‑Based Cellular Signatures (LINCS), we simultaneously probed transcriptomic signatures of putative COVID‑19 drugs and publicly available SARS‑CoV‑2 infected cell lines to identify novel therapeutics. We identifed a shortlist of 20 candidate drugs: 8 are already under trial for the treatment of COVID‑19, the remaining 12 have antiviral properties and 6 have antiviral efcacy against coronaviruses specifcally, in vitro. All candidate drugs are either FDA approved or are under investigation. Our candidate drug fndings are discordant with (i.e., reverse) SARS‑CoV‑2 transcriptome signatures generated in vitro, and a subset are also identifed in transcriptome signatures generated from COVID‑19 patient samples, like the MEK inhibitor selumetinib. Overall, our fndings provide additional support for drugs that are already being explored as therapeutic agents for the treatment of COVID‑19 and identify promising novel targets that are worthy of further investigation. -

Daiichi Sankyo Group Value Report 2019
External Evaluations (as of June 30,2019) ™ Daiichi Sankyo Group Value Report 2019 Value Daiichi Sankyo Group MSCI Japan Empowering Women Select Index THE INCLUSION OF DAIICHI SANKYO CO.,LTD. IN ANY MSCI INDEX, AND THE USE OF MSCI LOGOS, TRADEMARKS, SERVICE MARKS OR INDEX NAMES HEREIN, DO NOT CONSTITUTE A SPONSORSHIP, ENDORSEMENT OR PROMOTION OF DAIICHI SANKYO CO.,LTD. BY MSCI OR ANY OF ITS AFFILIATES. THE MSCI INDEXES ARE THE EXCLUSIVE PROPERTY OF MSCI. MSCI AND THE MSCI INDEX NAMES AND LOGOS ARE TRADE- MARKS OR SERVICE MARKS OF MSCI OR ITS AFFILIATES. “Eruboshi” Certification Mark “Kurumin” Certification Mark Logo given to Certified Health and Productivity Management Organization (White500) This report uses FSC® certified paper, which indicates that the paper used to print this Paper report was produced from properly managed forests. 3-5-1, Nihonbashi-honcho, Chuo-ku, Tokyo 103-8426, Japan This report was printed using 100% Inks biodegradable printing inks from vegetable Corporate Communications Department oil. Daiichi Sankyo Group Tel: +81-3-6225-1126 CSR Department The waterless printing method used for this Value Report 2019 Tel: +81-3-6225-1067 Printing report minimized the use and release of harmful liquid wastes. https://www.daiichisankyo.com/ Printed in Japan 005_7045687911909.indd 1 2019/09/27 18:22:19 Introduction Our Mission The Core Values and Commitments serve as the criteria for business activities and In addition, we have established the DAIICHI SANKYO Group Corporate Conduct Charter . decision-making used by executive officers and employees in working to fulfill Our Mission . This charter calls on us to fulfill our social responsibilities by acting with the highest ethical Our Corporate Slogan succinctly explains the spirit of Our Mission, Core Values and standards and a good social conscience appropriate for a company engaged in business Commitments. -

FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Wu et al. Journal of Hematology & Oncology (2018) 11:133 https://doi.org/10.1186/s13045-018-0675-4 REVIEW Open Access FLT3 inhibitors in acute myeloid leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2* Abstract FLT3 mutations are one of the most common findings in acute myeloid leukemia (AML). FLT3 inhibitors have been in active clinical development. Midostaurin as the first-in-class FLT3 inhibitor has been approved for treatment of patients with FLT3-mutated AML. In this review, we summarized the preclinical and clinical studies on new FLT3 inhibitors, including sorafenib, lestaurtinib, sunitinib, tandutinib, quizartinib, midostaurin, gilteritinib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG 925, TTT-3002, and FF-10101. New generation FLT3 inhibitors and combination therapies may overcome resistance to first-generation agents. Keywords: FMS-like tyrosine kinase 3 inhibitors, Acute myeloid leukemia, Midostaurin, FLT3 Introduction RAS, MEK, and PI3K/AKT pathways [10], and ultim- Acute myeloid leukemia (AML) remains a highly resist- ately causes suppression of apoptosis and differentiation ant disease to conventional chemotherapy, with a me- of leukemic cells, including dysregulation of leukemic dian survival of only 4 months for relapsed and/or cell proliferation [11]. refractory disease [1]. Molecular profiling by PCR and Multiple FLT3 inhibitors are in clinical trials for treat- next-generation sequencing has revealed a variety of re- ing patients with FLT3/ITD-mutated AML. In this re- current gene mutations [2–4]. New agents are rapidly view, we summarized the preclinical and clinical studies emerging as targeted therapy for high-risk AML [5, 6]. on new FLT3 inhibitors, including sorafenib, lestaurtinib, In 1996, FMS-like tyrosine kinase 3/internal tandem du- sunitinib, tandutinib, quizartinib, midostaurin, gilteriti- plication (FLT3/ITD) was first recognized as a frequently nib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG mutated gene in AML [7]. -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

BCBSVT Specialty Drug List Effective 2021.07.01.Xlsx
Effective Date: 07/01/2021 SPECIALTY DRUG LIST Revised Date: 05/07/2021 DOSAGE EXCLUDED ON NATIONAL DRUG CLASS DRUG NAME GENERIC NAME FORM PERFORMANCE FORMULARY ANEMIA ARANESP SOLN DARBEPOETIN ALFA SOLN INJ ANEMIA ARANESP SOSY DARBEPOETIN ALFA SOLN PREFILLED SYRINGE ANEMIA EPOGEN SOLN EPOETIN ALFA INJ X ANEMIA PROCRIT SOLN EPOETIN ALFA INJ X ANEMIA REBLOZYL SOLR LUSPATERCEPT-AAMT FOR SUBCUTANEOUS INJ ANEMIA RETACRIT SOLN EPOETIN ALFA-EPBX INJ ANTI-GOUT AGENT KRYSTEXXA SOLN PEGLOTICASE INJ (FOR IV INFUSION) ANTI-INFECTIVE PREVYMIS SOLN LETERMOVIR IV SOLN ANTI-INFECTIVE PREVYMIS TABS LETERMOVIR TAB ASTHMA CINQAIR SOLN RESLIZUMAB IV INFUSION SOLN ASTHMA FASENRA SOSY BENRALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE ASTHMA FASENRA PEN SOAJ BENRALIZUMAB SUBCUTANEOUS SOLN AUTO-INJECTOR ASTHMA NUCALA SOAJ MEPOLIZUMAB SUBCUTANEOUS SOLUTION AUTO-INJECTOR ASTHMA NUCALA SOLR MEPOLIZUMAB FOR INJ ASTHMA NUCALA SOSY MEPOLIZUMAB SUBCUTANEOUS SOLUTION PREF SYRINGE ASTHMA XOLAIR SOLR OMALIZUMAB FOR INJ ASTHMA XOLAIR SOSY OMALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE CARDIOVASCULAR VYNDAMAX CAPS TAFAMIDIS CAP CARDIOVASCULAR VYNDAQEL CAPS TAFAMIDIS MEGLUMINE (CARDIAC) CAP CENTRAL NERVOUS SYSTEM AGENTS AUSTEDO TABS DEUTETRABENAZINE TAB CENTRAL NERVOUS SYSTEM AGENTS ENSPRYNG SOSY SATRALIZUMAB-MWGE SUBCUTANEOUS SOLN PREF SYRINGE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ CAPS TASIMELTEON CAPSULE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ LQ SUSP TASIMELTEON ORAL SUSP CHEMOTHERAPY PROTECTANT AMIFOSTINE SOLR AMIFOSTINE CRYSTALLINE FOR INJ CHEMOTHERAPY PROTECTANT ELITEK -

Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling While Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells
Published OnlineFirst February 14, 2013; DOI: 10.1158/1535-7163.MCT-12-0305 Molecular Cancer Cancer Therapeutics Insights Therapeutics Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling while Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells Ruwanthi N. Gunawardane, Ronald R. Nepomuceno, Allison M. Rooks, Jeremy P. Hunt, Jill M. Ricono, Barbara Belli, and Robert C. Armstrong Abstract Fms-like tyrosine kinase 3 (FLT3) is implicated in the pathogenesis of acute myeloid leukemia (AML). FLT3-activating internal tandem duplication (ITD) mutations are found in approximately 30% of patients with AML and are associated with poor outcome in this patient population. Quizartinib (AC220) has previously been shown to be a potent and selective FLT3 inhibitor. In the current study, we expand on previous observations by showing that quizartinib potently inhibits the phosphorylation of FLT3 and downstream signaling molecules independent of FLT3 genotype, yet induces loss of viability only in cells expressing constitutively activated FLT3. We further show that transient exposure to quizartinib, whether in vitro or in vivo, leads to prolonged inhibition of FLT3 signaling, induction of apoptosis, and drastic reductions in tumor volume and pharmacodynamic endpoints. In vitro experiments suggest that these prolonged effects are mediated by slow binding kinetics that provide for durable inhibition of the kinase following drug removal/clearance. Together these data suggest quizartinib, with its unique combination of selectivity and potent/sustained inhibition of FLT3, may provide a safe and effective treatment against FLT3-driven leukemia. Mol Cancer Ther; 12(4); 1–10. Ó2013 AACR. Introduction downstream signaling cascades including the Ras/ Fms-like tyrosine kinase 3 (FLT3) is a member of the MAPK, PI3K/Akt, and STAT5 pathways (1, 9–13). -

ARCTURUS THERAPEUTICS HOLDINGS INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event reported): May 20, 2020 ARCTURUS THERAPEUTICS HOLDINGS INC. (Exact name of registrant as specified in its charter) Delaware 001-38942 32-0595345 (State or other jurisdiction (Commission (I.R.S. Employer of incorporation) File Number) Identification No.) 10628 Science Center Drive, Suite 250 San Diego, California 92121 (Address of principal executive offices) Registrant’s telephone number, including area code: (858) 900-2660 (Former name or former address, if changed since last report) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: ☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) ☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) ☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) ☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Securities registered pursuant to Section 12(b) of the Act: Trading Name of each exchange Title of each class Symbol(s) on which registered Common stock, par value $0.001 per share ARCT The NASDAQ Stock Market LLC Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). -
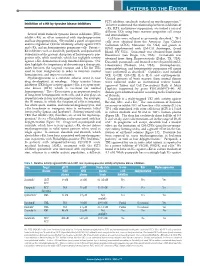
Letters to the Editor
LETTERS TO THE EDITOR FLT3 inhibitor, sorafenib, induced no myelosuppression.5,6 Inhibition of c-Kit by tyrosine kinase inhibitors To better understand the relationship between inhibition of c-Kit, FLT3, and marrow suppression, we studied a series of different TKIs using bone marrow progenitor cell assays Several small molecule tyrosine kinase inhibitors (TKIs) and immunoblots. inhibit c-Kit, an effect associated with myelosuppression Cell lines were cultured as previously described.2 TF-1 and hair depigmentation. We studied a panel of approved cells were obtained from the American Type Culture and investigational TKIs for inhibitory activity against FLT3 Collection (ATCC; Manassas, VA, USA) and grown in and c-Kit, and on hematopoietic progenitor cells. Potent c- RPMI supplemented with GM-CSF (Invitrogen, Grand Kit inhibitors such as dasatinib, pazopanib, and quizartinib Island, NY, USA). Quizartinib was obtained from Ambit demonstrated the greatest disruption of hematopoietic pro- Biosciences (San Diego, CA, USA). Crenolanib was genitor cells, while sorafenib, which has negligible activity obtained from Arog Pharmaceuticals (Dallas, TX, USA). against c-Kit, demonstrated only minimal disruption. Our Dasatinib, pazopanib, and imatinib were obtained from LC data highlight the importance of determining a therapeutic Laboratories (Woburn, MA, USA). Electrophoresis, index between the targeted receptor and c-Kit for TKIs immunoblotting, and hematopoietic progenitor cell assays used to treat malignancies in order to maintain normal were performed as described.2 Cytokines used included hematopoiesis and improve outcomes. SCF, G-CSF, GM-CSF, IL-3, IL-6, and erythropoietin. Myelosuppression is a common adverse event in new Unused portions of bone marrow from normal donors drug development in oncology.