Hydromorphone Trial JAMA Psychiatry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydromorphone
Hydromorphone WHAT IS HYDROMORPHONE? sedation, and reduced anxiety. It may also cause Hydromorphone belongs to a class of drugs mental clouding, changes in mood, nervousness, called “opioids,” which includes morphine. It and restlessness. It works centrally (in the has an analgesic potency of two to eight times brain) to reduce pain and suppress cough. greater than that of morphine and has a rapid Hydromorphone use is associated with both onset of action. physiological and psychological dependence. WHAT IS ITS ORIGIN? What is its effect on the body? Hydromorphone is legally manufactured and Hydromorphone may cause: distributed in the United States. However, • Constipation, pupillary constriction, urinary retention, users can obtain hydromorphone from nausea, vomiting, respiratory depression, dizziness, forged prescriptions, “doctor-shopping,” impaired coordination, loss of appetite, rash, slow or theft from pharmacies, and from friends and rapid heartbeat, and changes in blood pressure acquaintances. What are its overdose effects? What are the street names? Acute overdose of hydromorphone can produce: Common street names include: Severe respiratory depression, drowsiness • D, Dillies, Dust, Footballs, Juice, and Smack progressing to stupor or coma, lack of skeletal muscle tone, cold and clammy skin, constricted What does it look like? pupils, and reduction in blood pressure and heart Hydromorphone comes in: rate • Tablets, capsules, oral solutions, and injectable Severe overdose may result in death due to formulations respiratory depression. How is it abused? Which drugs cause similar effects? Users may abuse hydromorphone tablets by Drugs that have similar effects include: ingesting them. Injectable solutions, as well as • Heroin, morphine, hydrocodone, fentanyl, and tablets that have been crushed and dissolved oxycodone in a solution may be injected as a substitute for heroin. -

Do You Know... Cocaine
Do You Know... Street names: blow, C, coke, crack, flake, freebase, rock, snow Cocaine What is cocaine? Cocaine is a stimulant drug. Stimulants make people feel more alert and energetic. Cocaine can also make people feel euphoric, or “high.” Pure cocaine was first isolated from the leaves of the coca bush in 1860. Researchers soon discovered that cocaine numbs whatever tissues it touches, leading to its use as a local anesthetic. Today, we mostly use synthetic anesthetics, rather than cocaine. In the 1880s, psychiatrist Sigmund Freud wrote scientific papers that praised cocaine as a treatment for many ailments, including depression and alcohol and opioid addiction. After this, cocaine became widely and legally available in patent medicines and soft drinks. As cocaine use increased, people began to discover its dangers. In 1911, Canada passed laws restricting the importation, manufacture, sale and possession of cocaine. The use of 1/4 © 2003, 2010 CAMH | www.camh.ca cocaine declined until the 1970s, when it became known How does cocaine make you feel? for its high cost, and for the rich and glamorous people How cocaine makes you feel depends on: who used it. Cheaper “crack” cocaine became available · how much you use in the 1980s. · how often and how long you use it · how you use it (by injection, orally, etc.) Where does cocaine come from? · your mood, expectation and environment Cocaine is extracted from the leaves of the Erythroxylum · your age (coca) bush, which grows on the slopes of the Andes · whether you have certain medical or psychiatric Mountains in South America. -

Commonly Used Drugs
Commonly Used Drugs Many drugs can alter a person’s thinking and judgment, and can lead to health risks, including addiction, drugged driving, infectious disease, and adverse effects on pregnancy. Information on commonly used drugs with the potential for misuse or addiction can be found here. For information about treatment options for substance use disorders, see NIDA’s Treatment pages. For drug use trends, see our Trends and Statistics page. For the most up-to-date slang terms, please see Slang Terms and Code Words: A Reference for Law Enforcement Personnel (DEA, PDF, 1MB). The following drugs are included in this resource: ➢ Alcohol ➢ Methamphetamine ➢ Ayahuasca ➢ Over-the-Counter Medicines--Dextromethorphan ➢ Central Nervous System Depressants (DXM) ➢ Cocaine ➢ Over-the-Counter Medicines--Loperamide ➢ DMT ➢ PCP ➢ GHB ➢ Prescription Opioids ➢ Hallucinogens ➢ Prescription Stimulants ➢ Heroin ➢ Psilocybin ➢ Inhalants ➢ Rohypnol® (Flunitrazepam) ➢ Ketamine ➢ Salvia ➢ Khat ➢ Steroids (Anabolic) ➢ Kratom ➢ Synthetic Cannabinoids ➢ LSD ➢ Synthetic Cathinones ("Bath Salts") ➢ Marijuana (Cannabis) ➢ Tobacco/Nicotine ➢ MDMA (Ecstasy/Molly) ➢ Mescaline (Peyote) **Drugs are classified into five distinct categories or schedules “depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential.” More information and the most up-to-date scheduling information can be found on the Drug Enforcement Administration’s website. June 2020 Alcohol People drink to socialize, celebrate, and relax. Alcohol often has a strong effect on people—and throughout history, people have struggled to understand and manage alcohol’s power. Why does alcohol cause people to act and feel differently? How much is too much? Why do some people become addicted while others do not? The National Institute on Alcohol Abuse and Alcoholism is researching the answers to these and many other questions about alcohol. -

1 Impact of Opioid Agonists on Mental Health in Substitution
Impact of opioid agonists on mental health in substitution treatment for opioid use disorder: A systematic review and Bayesian network meta-analysis of randomized clinical trials Supplementary Table 1_ Specific search strategy for each database The following general combination of search terms, Boolean operators, and search fields were used where “*” means that any extension of that word would be considered: Title field [opium OR opiate* OR opioid OR heroin OR medication assisted OR substitution treatment OR maintenance treatment OR methadone OR levomethadone OR buprenorphine OR suboxone OR (morphine AND slow) OR diamorphine OR diacetylmorphine OR dihydrocodeine OR hydromorphone OR opium tincture OR tincture of opium OR methadol OR methadyl OR levomethadyl] AND Title/Abstract field [trial* OR random* OR placebo] AND All fields [depress* OR anxiety OR mental] Wherever this exact combination was not possible, a more inclusive version of the search strategy was considered. Database Search Strategy Ovid for EBM Reviews - Cochrane Central Register of (opium or opiate$ or opioid or heroin or medication Controlled Trials August 2018; Embase 1974 to assisted or substitution treatment or maintenance September 07, 2018; MEDLINE(R) and Epub Ahead treatment or methadone or levomethadone or of Print, In-Process & Other Non-Indexed Citations buprenorphine or suboxone or (morphine and slow) or and Daily 1946 to September 07, 2018 diamorphine or diacetylmorphine or dihydrocodeine or hydromorphone or opium tincture or tincture of opium or methadol or methadyl -

Hydromorphone and Morphine Are Not the Same Drug!
HYDROmorphone and Morphine are not the same drug! HYDROmorphone and morphine are deemed high risk/high alert medications. An Independent Double Check (IDC) must be done to avoid errors when administering all high risk drugs. Please refer to Clinical Practice Standard 1-20-6-3-260. HYDROmorphone and morphine need to knows: HYDROmorphone is 5 times more potent than morphine! (e.g., 1 mg of HYDROmorphone PO is equivalent to 5 mg of morphine PO) When administered via the subcutaneous route, these drugs are twice as strong! Therefore when converting between oral and subcutaneous routes half the dose. (10 mg of oral morphine = 5 mg of subcutaneous morphine) HYDROmorphone and morphine: The name game. Both drugs are used in the management of moderate to severe pain. HYDROmorphone and morphine come in different brand names and formulations. HYDROmorphone = Dilaudid (Injectable), Jurnista (SR), HYDROmorph Contin (SR) Morphine = Morphine Sulphate (Injectable), MS-IR, M.O.S. (Liquid/Tablet/SR), M-Eslon (SR), Statex (Liquid/Tablet/Supp.), Doloral (Liquid), MS Contin (SR), Kadian (SR) (SR=Slow Release CAP) *As per NH, use the generic names (NOT THE BRAND NAME), of these drugs in your documentation. Common side effects: 3 ‘B’s of opioid prescribing: Common: Nausea, Vomiting, Constipation, Sedation, Syncope, Xerostomia (Dry Mouth), If your client is on an opioid: Delirium, Restlessness, Urinary Retention. Do they have a Breakthrough? Less Common: Respiratory Depression, Do they have something for Barfing? Opioid-Induced Neurotoxicity, Myoclonus, Do they have something for their Bowels? Pruritis (itching). Opioid induced neurotoxicity: What does it look like? Opioid induced neurotoxicity is the result of a build up of toxic opioid metabolites in the blood stream. -

Do You Know... Methadone
Do You Know... Street names: juice, meth (also used to refer to methamphetamines) What is it? Methadone belongs to the opioid family of Methadone drugs. It is used most commonly to treat addiction to other opioid drugs such as heroin, oxycodone (e.g., Percodan, Percocet), fentanyl (e.g., Duragesic, Sublimaze) and hydromorphone (e.g., Dilaudid). Methadone is a synthetic opioid, which means that it is made from chemicals in a lab. Methadone was developed in Germany during the Second World War and was first used to provide pain relief. Methadone maintenance treatment, which prevents opioid withdrawal and reduces or eliminates drug cravings, was first developed in the 1960s. For many years, Canadian reg- ulations around the prescription of methadone were so restrictive that few doctors offered the treatment. People who wanted methadone treatment often had to wait months or years. 1/4 © 2003, 2011, 2012 CAMH | www.camh.ca In the 1990s, the need to reduce the harm of drug use with medical care, improves the chances of having a was more clearly recognized, and changes were made healthy baby. There are no known long-term effects of to make it easier for doctors to provide methadone methadone on the baby. treatment. People who inject opioid drugs regularly, and who are Methadone maintenance is not a “cure”: it is a treatment. HIV- or hepatitis C–positive, are enrolled in methadone Through treatment, people who are addicted to opioids treatment to help protect their health. Methadone receive the medical and social support they need to treatment also helps to prevent these infections from stabilize and improve their lives. -

Hydromorphone Hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID and DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS a POTENT SCHEDULE II CONTROLLED OPIOID AGONIST
DILAUDID® ORAL LIQUID and DILAUDID® TABLETS (hydromorphone hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID AND DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST. SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, OXYCODONE, FENTANYL, AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH. DESCRIPTION Proprietary name: DILAUDID ORAL LIQUID Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 5 MILLIGRAM LIQUID purified water, methylparaben, propylparaben, sucrose, glycerin, sodium (C42953) metabisulfite Proprietary name: DILAUDID TABLETS Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 2 MILLIGRAM TABLET D&C red #30 Lake dye, D&C yellow #10 Lake dye, lactose, magnesium (C42998) stearate, sodium metabisulfite 2 4 MILLIGRAM TABLET D&C yellow #10 Lake dye, lactose, magnesium stearate, sodium metabisulfite (C42998) 3 8 MILLIGRAM TABLET lactose anhydrous, magnesium stearate, sodium metabisulfite (C42998) DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3- hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: M.W. 321.8 Each 5 mL (1 teaspoon) of DILAUDID ORAL LIQUID contains 5 mg of hydromorphone hydrochloride. In addition, other ingredients include purified water, methylparaben, propylparaben, sucrose, and glycerin. DILAUDID ORAL LIQUID may contain traces of sodium metabisulfite. -
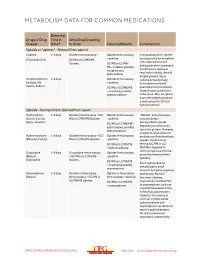
Metabolism Data for Common Medications
METABOLISM DATA FOR COMMON MEDICATIONS Detection Drugs or Drug Time in Urine Drug Screening Classes Urine* to Order Expected Results Consideration Opioids or “opiates” – Natural (from opium) Codeine 1–3 days Opiates Immunoassay + Opiates Immunoassay Immunoassays for “opiates” are responsive for morphine (Tylenol #2/3/4) GC/MS or LC/MS/MS – positive and codeine but do not Opiates GC/MS or LC/MS/ distinguish which is present. MS – codeine, possibly Confirmatory testing is morphine and required to reliably identify hydrocodone drug(s) present. Since Morphine (Avinza, 1–3 days Opiates Immunoassay codeine is metabolized Embeda, MS – positive to morphine and small Contin, Kadian) GC/MS or LC/MS/MS quantities to hydrocodone, – morphine, possibly these drugs may be found hydromorphone in the urine. Also, morphine may metabolize to produce a small amount (<10%) of hydromorphone. Opioids – Semisynthetic (derived from opium Hydrocodone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay “Opiates” immunoassays (Lorcet, Lortab, MS or LC/MS/MS Opiates – positive may also detect Norco, Vicodin) semisynthetic opioids GC/MS or LC/MS/MS – depending on their cross- hydrocodone, possibly reactivity pattern. However, hydromorphone a negative result does not Hydromorphone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay exclude use of semisynthetic (Dilaudid, Exalgo) MS or LC/MS/MS Opiates –positive opioids. Confirmatory GC/MS or LC/MS/MS testing (GC/MS or LC/ – hydromorphone MS/MS) is required to verify compliance with the Oxycodone 1–3 days Oxycodone Immunoassay Opiates Immunoassay prescribed semisynthetic (Roxicet, + GC/MS or LC/MS/MS –positive opioid(s). OxyContin) Opiates GC/MS or LC/MS/MS Since hydrocodone is – oxycodone possibly metabolized in small oxymorphone amounts to hydromorphone, Oxymorphone 1–3 days Opiates or Oxycodone Opiates or Oxycodone both may be found in (Opana) Immunoassay + GC/MS or Immunoassay – positive the urine. -

Cocaine Drugfacts
DrugFacts Revised Abril 2021 Cocaine DrugFacts What is cocaine? Cocaine is a powerfully addictive stimulant drug made from the leaves of the coca plant native to South America. Although health care providers can use it for valid medical purposes, such as local anesthesia for some surgeries, recreational cocaine use is illegal. As a street drug, cocaine looks like a fine, white, crystal powder. Street dealers often mix Photo ©Shutterstock/Africa Studio it with things like cornstarch, talcum powder, or flour to increase profits. They may also mix it with other drugs such as the stimulant amphetamine, or synthetic opioids, including fentanyl. Adding synthetic opioids to cocaine is especially risky when people using cocaine don’t realize it contains this dangerous additive. Increasing numbers of overdose deaths among cocaine users might be related to this tampered cocaine. How do people use cocaine? People snort cocaine powder through the nose, or they rub it into their gums. Others dissolve the Page 1 powder and inject it into the bloodstream. Some people inject a combination of cocaine and heroin, called a Speedball. Another popular method of use is to smoke cocaine that has been processed to make a rock crystal (also called "freebase cocaine"). The crystal is heated to produce vapors that are inhaled into the lungs. This form of cocaine is called Crack, which refers to the crackling sound of the rock as it's heated. Some people also smoke Crack by sprinkling it on marijuana or tobacco, and smoke it like a cigarette. People who use cocaine often take it in binges—taking the drug repeatedly within a short time, at increasingly higher doses—to maintain their high. -

Cocaine + Heroin) Self-Administration by Rhesus Monkeys
Neuropsychopharmacology (2007) 32, 1985–1994 & 2007 Nature Publishing Group All rights reserved 0893-133X/07 $30.00 www.neuropsychopharmacology.org Effects of d-Amphetamine and Buprenorphine Combinations on Speedball (Cocaine + Heroin) Self-Administration by Rhesus Monkeys ,1 1 Nancy K Mello* and S Stevens Negus 1 Alcohol and Drug Abuse Research Center, Harvard Medical School-McLean Hospital, Belmont, MA, USA The simultaneous i.v. administration of heroin and cocaine, called a ‘speedball,’ is often reported clinically, and identification of effective pharmacotherapies is a continuing challenge. We hypothesized that treatment with combinations of a monoamine releaser d-amphetamine, and a mu partial agonist, buprenorphine, might reduce speedball self-administration by rhesus monkeys. Speedballs (0.01 mg/kg/inj cocaine + 0.0032 mg/kg/inj heroin) and food (1 g banana-flavored pellets) were available during four daily sessions on a second-order schedule of reinforcement (fixed ratio (FR)2 (variable ratio (VR)16:S)). Monkeys were treated for 10 days with saline or ascending doses of d-amphetamine (0.0032–0.032 mg/kg/h) + buprenorphine (0.075 or 0.237 mg/kg/day) in combination. d-Amphetamine + both doses of buprenorphine produced an amphetamine dose-dependent decrease in speedball self-administration in comparison to the saline treatment baseline (Po0.01–0.001), but food-maintained responding did not change significantly. d-Amphetamine alone (0.032 mg/kg/h) significantly decreased both food (Po0.01) and speedball-maintained responding (Po0.05). During saline control treatment, speedball unit doses of 0.0032 mg/kg/inj cocaine + 0.001 mg/kg/inj heroin were at the peak of the speedball dose–effect curve. -

Hydromorphone Hydrochloride) C-II
NDA 19-034/S-018-Label Page 1 DILAUDID® and DILAUDID-HP® INJECTION 1 mg/mL, 2 mg/mL, 4mg/mL, and 10 mg/mL (hydromorphone hydrochloride) C-II WARNING: DILAUDID-HP (high potency, 10 mg/mL ampules and vials) is a more concentrated solution of hydromorphone than DILAUDID INJECTION, and is intended for use only in opioid-tolerant patients. Do not confuse DILAUDID-HP with standard parenteral formulations of DILAUDID or other opioids, as overdose and death could result. DILAUDID® INJECTION (1, 2, and 4 mg/mL ampules, sterile solution for parenteral administration) and DILAUDID-HP® contain hydromorphone, a potent Schedule II opioid agonist. Schedule II opioid agonists, including morphine, oxymorphone, hydromorphone, oxycodone, fentanyl and methadone, have the highest potential for abuse and risk of producing respiratory depression. Ethanol, other opioids, and other central nervous system depressants (e.g., sedative-hypnotics, skeletal muscle relaxants) can potentiate the respiratory- depressant effects of hydromorphone and increase the risk of adverse outcome, including death. DESCRIPTION DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: NDA 19-034/S-018-Label Page 2 M.W. 321.8 DILAUDID INJECTION is available in ampules for parenteral administration. Each 1 mL of sterile solution contains 1 mg, 2 mg, or 4 mg hydromorphone hydrochloride with 0.2% sodium citrate and 0.2% citric acid solution. DILAUDID INJECTION ampules are sterile. HIGH POTENCY DILAUDID (DILAUDID-HP) is available in AMBER ampules or single dose vials for intravenous (IV), subcutaneous (SC), or intramuscular (IM) administration. -

Session Xxiv Drug Combinations Hs172a R01/10 1
SESSION XXIV DRUG COMBINATIONS HS172A R01/10 1 SESSION XXIV DRUG COMBINATIONS Upon successfully completing this session the student will be able to: o Explain the prevalence of polydrug use among drug impaired subjects and identify common combinations of drugs abused by those subjects. o Describe the possible effects that combinations of drugs can produce on the major indicators of drug impairment. o Define the terms "Null", "Overlapping", "Additive" and "Antagonistic" as they relate to polydrug effects. o Identify the specific effects that are most likely to be observed in persons under the influence of particular drug combinations. HS172A R01/10 2 A. The Prevalence of Polydrug Use Studies have shown that polydrug use is on the rise throughout the country. In the Los Angeles Field Validation Study (1985), nearly three-quarters (72%) of the subject’s who were evaluated were found to have two or more drugs in their blood samples. The most familiar drug of all, alcohol, apparently is an especially popular "mixer" with other drugs. Alcohol routinely shows up in combination with virtually everything else, and often DREs encounter subject’s who have consumed alcohol along with two or more other drugs. Cannabis is another popular "mixer", and frequently shows up in combination with Cocaine, PCP and various other drugs. The "speedball", a combination of Cocaine and Heroin, remains popular, despite the well-publicized hazards of this particular mixture. Polydrug use among suspected drug impaired drivers continues to be very common. Data collected from DREs from throughout the U.S. and entered into the national DRE tracking database indicates that approximately 25% of all cases where toxicology was conducted resulted in two or more drug categories detected.