Download Author Version (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Amino Acid Metabolism, Urea Cycle Semester Iv, Paper Cc8
AMINO ACID METABOLISM, UREA CYCLE SEMESTER IV, PAPER CC8 UREA CYCLE + Some of the NH4 formed in the breakdown of amino acids is consumed in the biosynthesis of nitrogen compounds. Most aquatic species, such as the bony fishes, are ammonotelic, excreting amino nitrogen as ammonia. The toxic ammonia is simply diluted in the surrounding water. Most terrestrial animals are ureotelic, excreting amino nitrogen in the form of urea; birds and reptiles are uricotelic, excreting amino nitrogen as uric acid. In ureotelic organisms, the ammonia deposited in the mitochondria of hepatocytes is converted to urea in the urea cycle. This pathway was discovered in 1932 by Hans Krebs. The urea cycle begins inside liver mitochondria, but three of the subsequent steps take place in the cytosol. The first amino group to enter the urea cycle is derived from ammonia in the mitochondrial matrix arising from various amino acids. The urea cycle begins with the coupling of free NH4 + with HCO3- to form carbamoyl phosphate. The synthesis of carbamoyl phosphate, though a simple molecule, is complex, comprising three steps, all catalyzed by carbamoyl phosphate synthetase. - The reaction begins with the phosphorylation of HCO3 to form carboxyphosphate, which then reacts with ammonium ion to form carbamic acid. Finally, a second molecule of ATP phosphorylates carbamic acid to carbamoyl phosphate. The consumption of two molecules of ATP makes this synthesis of carbamoyl phosphate essentially irreversible. Next, the carbamoyl group of carbamoyl phosphate, which has a high transfer potential because of its anhydride bond, is transferred to ornithine to form citrulline, in a reaction catalyzed by + ornithine transcarbamoylase. -

Ammonium Carbamate Hazard Summary Identification
Common Name: AMMONIUM CARBAMATE CAS Number: 1111-78-0 RTK Substance number: 0091 DOT Number: NA 9083 Date: January 1996 Revision: March 2002 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Ammonium Carbamate can affect you when breathed in. The following exposure limits are for Ammonia: * Exposure can irritate the skin and eyes causing redness and tearing. OSHA: The legal airborne permissible exposure limit * Breathing Ammonium Carbamate can irritate the nose, (PEL) is 50 ppm averaged over an 8-hour throat and lungs causing coughing and/or shortness of workshift. breath. NIOSH: The recommended airborne exposure limit is IDENTIFICATION 25 ppm averaged over a 10-hour workshift and Ammonium Carbamate is a white crystalline (sand-like) 35 ppm, not to be exceeded during any 15 minute powder. It is used as a fertilizer and ammoniating agent. work period. REASON FOR CITATION ACGIH: The recommended airborne exposure limit is 25 ppm averaged over an 8-hour workshift and * Ammonium Carbamate is on the Hazardous Substance 35 ppm as a STEL (short-term exposure limit). List because it is cited by DOT and EPA. * Definitions are provided on page 5. WAYS OF REDUCING EXPOSURE HOW TO DETERMINE IF YOU ARE BEING * Where possible, enclose operations and use local exhaust ventilation at the site of chemical release. If local exhaust EXPOSED ventilation or enclosure is not used, respirators should be The New Jersey Right to Know Act requires most employers worn. to label chemicals in the workplace and requires public * Wear protective work clothing. employers to provide their employees with information and * Wash thoroughly immediately after exposure to training concerning chemical hazards and controls. -

TR-541: Formamide (CASRN 75-12-7) in F344/N Rats and B6C3F1 Mice (Gavage Studies)
NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF FORMAMIDE (CAS NO. 75-12-7) IN F344/N RATS AND B6C3F1 MICE (GAVAGE STUDIES) NATIONAL TOXICOLOGY PROGRAM P.O. Box 12233 Research Triangle Park, NC 27709 July 2008 NTP TR 541 NIH Publication No. 08-5884 National Institutes of Health Public Health Service U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES FOREWORD The National Toxicology Program (NTP) is an interagency program within the Public Health Service (PHS) of the Department of Health and Human Services (HHS) and is headquartered at the National Institute of Environmental Health Sciences of the National Institutes of Health (NIEHS/NIH). Three agencies contribute resources to the program: NIEHS/NIH, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention (NIOSH/CDC), and the National Center for Toxicological Research of the Food and Drug Administration (NCTR/FDA). Established in 1978, the NTP is charged with coordinating toxicological testing activities, strengthening the science base in toxicology, developing and validating improved testing methods, and providing information about potentially toxic substances to health regulatory and research agencies, scientific and medical communities, and the public. The Technical Report series began in 1976 with carcinogenesis studies conducted by the National Cancer Institute. In 1981, this bioassay program was transferred to the NTP. The studies described in the Technical Report series are designed and conducted to characterize and evaluate the toxicologic potential, including carcinogenic activity, of selected substances in laboratory animals (usually two species, rats and mice). Substances selected for NTP toxicity and carcinogenicity studies are chosen primarily on the basis of human exposure, level of production, and chemical structure. -

Federal Register / Vol. 62, No. 116 / Tuesday, June 17, 1997 / Rules and Regulations
32974 Federal Register / Vol. 62, No. 116 / Tuesday, June 17, 1997 / Rules and Regulations ENVIRONMENTAL PROTECTION p.m. EST, toll-free, at 800±424±9346; standards for owners and operators of AGENCY 703±412±9810 from Government phones hazardous waste treatment, storage and or if in the Washington, DC local calling disposal facilitiesÐincluding standards 40 CFR Parts 261, 268, 271, and 302 area; or 800±553±7672 for the hearing for obtaining permitsÐare found at 40 impaired. For more detailed information CFR part 264. [EPA530±Z±97±FFF; FRL±5839±7] on specific aspects of the rulemaking, Hazardous wastes are also subject to RIN 2050±AD59 contact Caroline Gerwe by calling 703± land disposal restrictions under 40 CFR 308±3540 or by writing, to U.S. part 268. EPA's authorizations for state Hazardous Waste Management Environmental Protection Agency, hazardous waste programs are found at System; Carbamate Production, Office of Solid Waste, Hazardous Waste 40 CFR part 272. The requirements for Identification and Listing of Hazardous Identification Division, 401 M St., SW., obtaining these authorizations are found Waste; Land Disposal Restrictions; (Mailcode 5304W), Washington, DC at 40 CFR part 271. Authorization of State Hazardous 20460. In addition, hazardous wastes having Waste Programs; and CERCLA SUPPLEMENTARY INFORMATION: This rule the characteristics identified under, or Hazardous Substance Designation and is available on the Internet. Please listed pursuant to, RCRA section 3001 Reportable Quantities follow these instructions to access the (except when suspended by Congress) AGENCY: Environmental Protection rule electronically: From the World become hazardous substances under Agency (EPA). Wide Web (WWW), type http:// section 101(14)(C) of CERCLA, 42 U.S.C. -
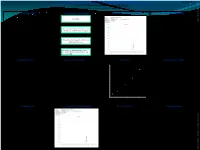
Quantification of Ethyl Carbamate in Tobacco
QUANTIFICATION OF ETHYL CARBAMATE IN TOBACCO POSTER-15 Dinesh.T.K., Sathya Gourishankar and Sharad.K.Mehta* ITC Ltd, ITC R&D Centre, Peenya, Bangalore – 560 058, INDIA. ABSTRACT SAMPLE PREPARATION SAMPLE CHROMATOGRAM REPRODUCIBILITY ETHYL 2.5g Sample CARBAMATE ANALYST TRIAL NO. SAMPLE (ng/g) 2012_TSRC15_Mehta.pdf A simple method is developed for quantification of Ethyl 1 Tobacco 371.0 carbamate in Tobacco. Ethyl carbamate in Tobacco is 2 Tobacco 368.7 extracted with water and is partitioned with Sonicate with 25ml water for 30 mins and 3 Tobacco 382.2 dichloromethane. The dichloromethane extract is Centrifuge at 10000 rpm for 10 minutes. 4 Tobacco 374.1 concentrated and injected in GC/MS-SIM mode. The 1 5 Tobacco 384.1 method has been validated using standard validation 6 Tobacco 389.0 protocols and there is excellent linearity over 7 Tobacco 368.3 concentration range from 100 to 4000 ng/g with Extract 10 ml of water extract with 30ml correlation coefficient of 0.9939. The recoveries are more dichloromethane twice. 8 Tobacco 367.1 than 90% for Ethyl carbamate with limit of quantification 9 Tobacco 364.5 100 ng/g and limit of detection 40ng/g respectively. carbamate Ethyl 2 10 Tobacco 372.2 Concentrate the dichloromethane extract STDEV 8.2 after passing through sodium sulphate and MEAN 374.1 inject in GC-MS. %RSD 2.2 INTRODUCTION GC-MS CONDITIONS LINEARITY RECOVERY STUDIES CALIBRATION FOR ETHYL CARBAMATE 400000 Ethyl carbamate (Urethane) is a genotoxic carcinogen found 350000 in fermented foods and beverages. Ethyl carbamate in y = 340.35x - 5869.2 SPIKED OBTAINED Tobacco is formed from Maleic hydrazide, which is a plant • Column : Zebron-ZB Wax plus 30mX0.25mmX0.25m. -

Safety Data Sheet
SAFETY DATA SHEET Date Printed: November 30, 2016 Version: 10 Regulation: According to Regulation 2012 OSHA Hazard Communication Standard; 29 CFR Part 1910.1200 1. Identification 1.1 Product identifier 1.1.1 Product name: KONNATE L-75 1.1.2 Other means of identification: - 1.2 Recommended use of the chemical and restrictions on use 1.2.1 Recommended use: Wood, metal, synthetic leather, adhesives, curing agents, inks, coatings, etc. 1.2.2. Restrictions on use: Do not use for purposes other than those recommended. 1.3 Details of the supplier of the safety data sheet 1.3.1 Manufacturer Company name: TDI Plant, Hanwha Chemical Co, Ltd. Address: 46-47, Yeosusandan 2-ro, Yeosu-si, Jeollanam-do, Korea Prepared by: TDI Production Team Contact Telephone: +82-61-688-4800 1.3.2 Supplier & Distributor Company name: Hanwha Chemical Co, Ltd. Address: 18F Hanwha Bldg., Janggyo-dong, Jung-gu, Seoul, Korea Prepared by: TDI Sales Team Contact Telephone: +82-2-729-2700 1.4 Emergency phone number Emergency phone : 1-800-424-9300, +1 703-527-3887 (Any problems that occures in U.S.A) 2. Hazard(s) identification 2.1 Classification of the substance or mixture According to Regulation 2012 OSHA Hazard Communication Standard; 29 CFR Part 1910.1200 Physical / Chemical Hazards: Flammable liquids: Category 2 Health Hazards: Specific target organ toxicity (Single exposure): Category 3 (respiratory tract irritation) Environmental Hazards: Not classified 2.2 Label elements, including precautionary statements ο Pictogram and symbol: ο Signal word: Danger ο Hazard statements: H225 Highly flammable liquid and vapors H335 May cause respiratory irritation. -

Carbamic Acid Produced by the UV/EUV Irradiation of Interstellar Ice Analogs
A&A 464, 253–257 (2007) Astronomy DOI: 10.1051/0004-6361:20066631 & c ESO 2007 Astrophysics Carbamic acid produced by the UV/EUV irradiation of interstellar ice analogs Y. -J. C hen 1,M.Nuevo1,J.-M.Hsieh1,T.-S.Yih1,W.-H.Sun2,W.-H.Ip2, H.-S. Fung3,S.-Y.Chiang3,Y.-Y.Lee3,J.-M.Chen3,andC.-Y.R.Wu4 1 Department of Physics, National Central University, No. 300, Jhongda Rd, Jhongli City, Taoyuan County 32054, Taiwan e-mail: [email protected]; [email protected] 2 Graduate Institute of Astronomy, National Central University, No. 300, Jhongda Rd, Jhongli City, Taoyuan County 32049, Taiwan 3 National Synchrotron Radiation Research Center, Hsinchu 30076, Taiwan 4 Space Sciences Center and Department of Physics and Astronomy, University of Southern California, Los Angeles, CA 90089-1341, USA Received 24 October 2006 / Accepted 14 December 2006 ABSTRACT Context. Carbamic acid (NH2COOH) is the smallest amino acid, smaller than the smallest proteinaceous amino acid glycine. This compound has never been observed in the interstellar medium (ISM). Previous experiments where ice mixtures containing H2O, CO2 + − and NH3 were subjected to 1-MeV proton bombardment showed that carbamic acid is formed in a stable zwitterionic (NH3 COO ) form. 12 13 Aims. In the present work, we have carried out irradiations of ice mixtures containing H2O, CO2/ CO2 and NH3 with ultraviolet (UV)/extreme ultraviolet (EUV) photons provided by a synchrotron source in the 4–20 eV range, and compared the results with those obtained for energetic protons. Methods. Infrared (IR) spectroscopy and mass spectrometry were used to identify the formed photo-products and monitor their evolution in the ices at 15 K and during the warming up to room temperature in the formed residues. -

24Amino Acids, Peptides, and Proteins
WADEMC24_1153-1199hr.qxp 16-12-2008 14:15 Page 1153 CHAPTER COOϪ a -h eli AMINO ACIDS, x ϩ PEPTIDES, AND NH3 PROTEINS Proteins are the most abundant organic molecules 24-1 in animals, playing important roles in all aspects of cell structure and function. Proteins are biopolymers of Introduction 24A-amino acids, so named because the amino group is bonded to the a carbon atom, next to the carbonyl group. The physical and chemical properties of a protein are determined by its constituent amino acids. The individual amino acid subunits are joined by amide linkages called peptide bonds. Figure 24-1 shows the general structure of an a-amino acid and a protein. α carbon atom O H2N CH C OH α-amino group R side chain an α-amino acid O O O O O H2N CH C OH H2N CH C OH H2N CH C OH H2N CH C OH H2N CH C OH CH3 CH2OH H CH2SH CH(CH3)2 alanine serine glycine cysteine valine several individual amino acids peptide bonds O O O O O NH CH C NH CH C NH CH C NH CH C NH CH C CH3 CH2OH H CH2SH CH(CH3)2 a short section of a protein a FIGURE 24-1 Structure of a general protein and its constituent amino acids. The amino acids are joined by amide linkages called peptide bonds. 1153 WADEMC24_1153-1199hr.qxp 16-12-2008 14:15 Page 1154 1154 CHAPTER 24 Amino Acids, Peptides, and Proteins TABLE 24-1 Examples of Protein Functions Class of Protein Example Function of Example structural proteins collagen, keratin strengthen tendons, skin, hair, nails enzymes DNA polymerase replicates and repairs DNA transport proteins hemoglobin transports O2 to the cells contractile proteins actin, myosin cause contraction of muscles protective proteins antibodies complex with foreign proteins hormones insulin regulates glucose metabolism toxins snake venoms incapacitate prey Proteins have an amazing range of structural and catalytic properties as a result of their varying amino acid composition. -

Production of Carbamic Acid Dimer from Ammonia-Carbon Dioxide Ices: Matching Observed and Computed IR Spectra
Article Production of Carbamic Acid Dimer from Ammonia-Carbon Dioxide Ices: Matching Observed and Computed IR Spectra Zikri Altun 1, Erdi Bleda 2 and Carl Trindle 3,* 1 Physics Department, Marmara University, Istanbul 34722, Turkey; [email protected] 2 Physics Department, Marmara University, Istanbul 34722, Turkey; [email protected] 3 Chemistry Department, University of Virginia, Charlottesville 22902, VA, USA * Correspondence: [email protected]; Tel: +1-434-770-9197 Received: 20 February 2019; Accepted: 19 April 2019; Published: 23 April 2019 Abstract: The production of complex molecules in ammonia–carbon dioxide ices is presumed to pass through species of formula H3N:CO2 with further addition of ammonia and carbon dioxide. One possible landmark, carbamic acid, H2NCOOH, has been implicated among the products of warming and irradiation of such ices. Experimental study of the IR spectra of residues has suggested the presence of related species, including weakly bound 1:1 and 2:1 complexes of ammonia with carbon dioxide, zwitterionic carbamic acid, ammonium carbamate, and the dimer of carbamic acid. We computed the energetics and vibrational spectra of these species as well as the complex between ammonia and carbamic acid for gas and condensed phases. By means of a new spectrum-matching scoring between computed and observed vibrational spectra, we infer species that are most probably present. The leading candidates are ammonium carbamate, the carbamic acid–ammonia complex, and the carbamic acid dimer. Keywords: prebiotic chemistry; carbamic acid; carbon dioxide-ammonia ices; infrared spectra; anharmonicity 1. Experimental Evidence for Carbamic Acid The evolution of complex and perhaps biologically relevant molecules from the simple molecules well established to exist in the interstellar medium is a central issue in astrochemistry [1]. -

Historical Review: ATP As a Neurotransmitter
Review TRENDS in Pharmacological Sciences Vol.27 No.3 March 2006 Historical review: ATP as a neurotransmitter Geoffrey Burnstock Autonomic Neuroscience Centre, Royal Free & University College Medical School, Rowland Hill Street, London NW3 2PF, UK Purinergic signalling is now recognized to be involved in describing the physiological significance, pharmacological a wide range of activities of the nervous system, action and therapeutic use of adenylyl compounds in including neuroprotection, central control of autonomic humans was published in 1957 by Boettge et al. [8]. functions, neural–glial interactions, control of vessel Subsequently, an influential hypothesis was proposed by tone and angiogenesis, pain and mechanosensory Berne [9], who postulated that adenosine was the transduction and the physiology of the special senses. physiological mediator of the coronary vasodilatation In this article, I give a personal retrospective of the associated with myocardial hypoxia. An alternative discovery of purinergic neurotransmission in the early hypothesis was proposed later [10], namely that ATP 1970s, the struggle for its acceptance for w20 years, the released from endothelial cells during hypoxia and shear expansion into purinergic cotransmission and its event- stress acted on endothelial P2 receptors, resulting in the ual acceptance when receptor subtypes for ATP were release of nitric oxide (NO) and subsequent vasodilatation; cloned and characterized and when purinergic synaptic adenosine participated only in the longer-lasting com- transmission between neurons in the brain and periph- ponent of reactive hyperaemia (increased blood flow). eral ganglia was described in the early 1990s. I also Details of various early studies that reported extracellular discuss the current status of the field, including recent roles of ATP are summarized in a historical review interest in the pathophysiology of purinergic signalling published in 1997 [11]. -

Carbamic Acid Esters As N-Acylethanolamine
Article pubs.acs.org/jmc N-(2-Oxo-3-oxetanyl)carbamic Acid Esters as N-Acylethanolamine Acid Amidase Inhibitors: Synthesis and Structure−Activity and Structure−Property Relationships † † † ‡ ‡ Andrea Duranti, Andrea Tontini, Francesca Antonietti, Federica Vacondio, Alessandro Fioni, ‡ ‡ ‡ § § ∥ † Claudia Silva, Alessio Lodola, Silvia Rivara, Carlos Solorzano, Daniele Piomelli, , Giorgio Tarzia, ‡ and Marco Mor*, † Dipartimento di Scienze Biomolecolari, Universitàdegli Studi di Urbino “Carlo Bo”, Piazza del Rinascimento 6, I-61029 Urbino, Italy ‡ Dipartimento Farmaceutico, Universitàdegli Studi di Parma, Viale G. P. Usberti 27/A, I-43124 Parma, Italy § Department of Pharmacology, University of California, Irvine, 360 MSRII, California 92697-4625, United States ∥ Department of Drug Discovery and Development, Italian Institute of Technology, via Morego 30, I-16163 Genova, Italy ABSTRACT: The β-lactone ring of N-(2-oxo-3-oxetanyl)amides, a class of N-acylethanolamine acid amidase (NAAA) inhibitors endowed with anti-inflammatory properties, is responsible for both NAAA inhibition and low compound stability. Here, we investigate the structure−activity and structure−property relationships for a set of known and new β-lactone derivatives, focusing on the new class of N-(2-oxo-3-oxetanyl)carbamates. Replacement of the amide group with a carbamate one led to different stereoselectivity for NAAA inhibition and higher intrinsic stability, because of the reduced level of intramolecular attack at the lactone ring. The introduction of a syn methyl at the β-position of the lactone further improved chemical stability. A tert-butyl substituent in the side chain reduced the reactivity with bovine serum albumin. (2S,3R)-2-Methyl-4-oxo-3-oxetanylcarbamic acid 5-phenylpentyl ester (27, URB913/ARN077) inhibited NAAA with good in vitro potency (IC50 = 127 nM) and showed improved stability.