Study of Arsenic in Drinking Water of Distric Kasur Pakistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

8. TMA Kasur 17-04-17.Pdf
AUDIT REPORT ON THE ACCOUNTS OF TEHSILMUNICIPALADMINISTRATIONS DISTRICT KASUR AUDIT YEAR 2015-16 AUDITOR GENERAL OF PAKISTAN 1 TABLE OF CONTENTS ABBREVIATIONS AND ACRONYMS ................................................ i PREFACE .............................................................................................. ii EXECUTIVE SUMMARY ................................................................... iii Table 1: Audit Work Statistics ...............................................................vi Table 2: Audit observation regarding Financial Management .................vi Table 3: Outcome Statistics .................................................................. vii Table 4: Irregularities pointed out........................................................ viii Table 5: Cost-Benefit .......................................................................... viii 1.1 TEHSIL MUNICIPAL ADMINISTRATIONS, DISTRICT KASUR .................................................................................................. 1 1.1.1 Introduction ................................................................................. 1 1.1.2 Comments on Budget and Accounts (Variance Analysis)............. 2 1.1.3 Brief Comments on the Status of Compliance on MFDAC Audit Paras of Audit Report 2014-15 ................................................................ 3 1.1.4 Brief Comments on Status of Compliance with PAC Directives... 3 1.2 TMA KASUR ............................................................................ 5 1.2.1 Fraud / -

Short Communication Surveying Tubewell Water Suitability for Irrigation in Four Tehsils of District Kasur
Soil Environ. 30(2): 155-159, 2011 www.se.org.pk Online ISSN: 2075-1141 Print ISSN: 2074-9546 Short Communication Surveying tubewell water suitability for irrigation in four tehsils of district Kasur Ijaz Mehboob*1, Muhammad Siddique Shakir1 and Asrar Mahboob2 1Soil and Water Testing Laboratory, Kasur, Punjab, Pakistan 2Maize and Millets Research Institute, Yousaf Wala, Sahiwal, Punjab, Pakistan Abstract Four tehsils of district Kasur (Chunian, Pattoki, Kot Radha Kishan and Kasur) were surveyed and five villages were selected in each tehsil at random. Two water samples were collected from each village and were analyzed for various irrigation water quality parameters. The results indicated that 60% tubewell were unfit from Chunian, 90% from Pattoki, 90% from Kot Radha Kishan and 80% from Kasur tehsil. Overall, 20% of total tubewells water sampled had quality parameters within the acceptable limits whereas 80% were unfit for irrigation. About 97% waters were unfit due to high salinity (EC > 1250 mS cm-1), 63% were due to high sodium adsorption ratio (SAR > 10 mmol L-1)1/2 and 97% were due to high residual sodium carbonate (RSC > 2.5 me L-1). It may be inferred that use of poor quality irrigation water will cause deterioration in soil health, which consequently will result in poor crop production. Hence, it is emphasized that tubewell discharging unfit water should be used by following sound management practices like precision land leveling, inclusion of high salt tolerant crops in traditional cropping system, occasional deep ploughing in heavy textured soil, occasional flushing of the soil profile with heavy irrigation to reduce the salt concentration in the root zone and application of organic and inorganic amendments like pressmud, poultry manure, farm yard manure and gypsum or acid/acid formers etc, however the management options must be on the basis of analysis of water quality parameters. -

Quarterly Activity Report
Quarterly Activity Report October-December 2014 By Human and Institutional Development Section (HID) DAMEN 26-C Nawab Town Raiwind Road Lahore. Phone: 042-35310571-2, Fax: 042-35310473, URL: www.damen-pk.org, E-mail:[email protected] Quarterly Activity Report Oct-De c 2014 Written By Aisha Almass – Research & Documentation Officer Edited By Rukhshanda Riaz – Manager Human & Institutional Development (HID) PAGE 1 OF 21 Quarterly Activity Report Oct-De c 2014 Table of Contents Quarter Overview ....................................................................................................................................................... 3 Social Sector Program ................................................................................................................................................. 4 Home School Education Program ....................................................................................................................... 4 Health Care services............................................................................................................................................. 5 Health Camp Campaign ....................................................................................................................................... 7 Environmental Consciousness............................................................................................................................. 8 Community Transformation ............................................................................................................................... -

Find Address of Your Nearest Loan Center and Phone Number of Concerned Focal Person
Find address of your nearest loan center and phone number of concerned focal person Loan Center/ S.No. Province District PO Name City / Tehsil Focal Person Contact No. Union Council/ Location Address Branch Name Akhuwat Islamic College Chowk Oppsite Boys College 1 Azad Jammu and Kashmir Bagh Bagh Bagh Nadeem Ahmed 0314-5273451 Microfinance (AIM) Sudan Galli Road Baagh Akhuwat Islamic Muzaffarabad Road Near main bazar 2 Azad Jammu and Kashmir Bagh Dhir Kot Dhir Kot Nadeem Ahmed 0314-5273451 Microfinance (AIM) dhir kot Akhuwat Islamic Mang bajri arja near chambar hotel 3 Azad Jammu and Kashmir Bagh Harighel Harighel Nadeem Ahmed 0314-5273451 Microfinance (AIM) Harighel Akhuwat Islamic 4 Azad Jammu and Kashmir Bhimber Bhimber Bhimber Arshad Mehmood 0346-4663605 Kotli Mor Near Muslim & School Microfinance (AIM) Akhuwat Islamic 5 Azad Jammu and Kashmir Bhimber Barnala Barnala Arshad Mehmood 0346-4663605 Main Road Bimber & Barnala Road Microfinance (AIM) Akhuwat Islamic Main choki Bazar near Sir Syed girls 6 Azad Jammu and Kashmir Bhimber Samahni Samahni Arshad Mehmood 0346-4663605 Microfinance (AIM) College choki Samahni Helping Hand for Adnan Anwar HHRD Distrcict Office Relief and Hattian,Near Smart Electronics,Choke 7 Azad Jammu and Kashmir Hattian Hattian UC Hattian Adnan Anwer 0341-9488995 Development Bazar, PO, Tehsil and District (HHRD) Hattianbala. Helping Hand for Adnan Anwar HHRD Distrcict Office Relief and Hattian,Near Smart Electronics,Choke 8 Azad Jammu and Kashmir Hattian Hattian UC Langla Adnan Anwer 0341-9488995 Development Bazar, PO, Tehsil and District (HHRD) Hattianbala. Helping Hand for Relief and Zahid Hussain HHRD Lamnian office 9 Azad Jammu and Kashmir Hattian Hattian UC Lamnian Zahid Hussain 0345-9071063 Development Main Lamnian Bazar Hattian Bala. -

Renewal List
Renewal List S/NO REN# / NAME FATHER'S NAME PRESENT ADDRESS DATE OF ACADEMIC REN DATE BIRTH QUALIFICATION 1 32179 NAVEED ASIF SIBGATULLAH CHOUNIAN ROAD OLD MANDI PATTOKI 4-10-1980 MATRIC 11/07/2014 DISTT. KASUR , KASUR, PUNJAB 2 31791 MUHAMMAD MUHAMMAD MUSHTAQ MOH, BAGECHI MUSTAFABAD DISTT. KASUR 12-12- MATRIC 11/07/2014 IMRAN ALI , KASUR, PUNJAB 1985 3 41634 MUHAMMAD KHUSHI MUHAMMAD UNITED PHARMACY USMAN WALA DISTT. 15-5-1969 MATRIC 11/7/2014 ASHRAF KASUR , KASUR, PUNJAB 4 26266 REHANA AZMAT AZMAT ULLAH H/NO. 90/C MAIN ROAD MALIK PURA 20-10- MATRIC 11/07/2014 PATTOKI DISTT. KASUR , KASUR, PUNJAB 1964 5 37165 GHULAM GHAUS ARSHAD MEHMOOD LAMBAY JAGIR P/O SAME TEH, PATTOKI 3-4-1985 MATRIC 11/7/2014 DISTT. KASUR, KASUR, PUNJAB 6 29368 SHABANA SHAH SYED SHABEER HASAN ST. YOUSAFSHAH KOT GHULAM 2-3-1976 MATRIC 11/07/2014 SHAH MUHAMMAD NEAR MADINA MASJID KASUR , KASUR, PUNJAB 7 48167 MUHAMMAD ABDUL RAZZAQ ST. MIAN JALAL TOLU WALI NAI ABADI 1-11-1983 MATRIC 11/07/2014 AFZAL BHAHSAR BURA , KASUR, PUNJAB 8 25431 MUHAMMAD MUHAMMAD ANWAR H.NO. 9 ST, NO. 9 MOH, MALIK PURA TEH 14-2-1982 MATRIC 11/07/2014 SARWAR ANWAR PATTOKI DISTT. KASUR., KASUR, PUNJAB 9 34058 JAVED SOHRAB KHAN KOT BAWA CHAK NO. 24, TEH, PATTOKI 4-1-1966 MATRIC 12/07/2014 MUHAMMAD DISTT. KASUR , KASUR, PUNJAB 10 25765 QUDRAT ULLAH ABDUL GHUFOOR MOH, BALOCHAN SHAIUL TOWN KHUDIAN 1-1-1983 MATRIC 12/07/2014 KHAS DISTT. KASUR , KASUR, PUNJAB 11 26244 MUHAMMAD GULZAR ALI VILL VERAN HATTER P/O KHUNDIAN KASUR 7-7-1970 MATRIC 12/07/2014 ASLAM , KASUR, PUNJAB 12 37348 BABAR KHALIL AHMAD BALOUI P/O SAME TEH, PATTOKI DISTT. -

Unclaimed Deposit 2014
Details of the Branch DETAILS OF THE DEPOSITOR/BENEFICIARIYOF THE INSTRUMANT NAME AND ADDRESS OF DEPOSITORS DETAILS OF THE ACCOUNT DETAILS OF THE INSTRUMENT Transaction Federal/P rovincial Last date of Name of Province (FED/PR deposit or in which account Instrume O) Rate Account Type Currency Rate FCS Rate of withdrawal opened/instrume Name of the nt Type In case of applied Amount Eqv.PKR Nature of Deposit ( e.g Current, (USD,EUR,G Type Contract PKR (DD-MON- Code Name nt payable CNIC No/ Passport No Name Address Account Number applicant/ (DD,PO, Instrument NO Date of issue instrumen date Outstandi surrender (LCY,UFZ,FZ) Saving, Fixed BP,AED,JPY, (MTM,FC No (if conversio YYYY) Purchaser FDD,TDR t (DD-MON- ng ed or any other) CHF) SR) any) n , CO) favouring YYYY) the Governm ent 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 PRIX 1 Main Branch Lahore PB Dir.Livestock Quetta MULTAN ROAD, LAHORE. 54500 LCY 02011425198 CD-MISC PHARMACEUTICA TDR 0000000189 06-Jun-04 PKR 500 12-Dec-04 M/S 1 Main Branch Lahore PB MOHAMMAD YUSUF / 1057-01 LCY CD-MISC PKR 34000 22-Mar-04 1 Main Branch Lahore PB BHATTI EXPORT (PVT) LTD M/S BHATTI EXPORT (PVT) LTD M/SLAHORE LCY 2011423493 CURR PKR 1184.74 10-Apr-04 1 Main Branch Lahore PB ABDUL RAHMAN QURESHI MR ABDUL RAHMAN QURESHI MR LCY 2011426340 CURR PKR 156 04-Jan-04 1 Main Branch Lahore PB HAZARA MINERAL & CRUSHING IND HAZARA MINERAL & CRUSHING INDSTREET NO.3LAHORE LCY 2011431603 CURR PKR 2764.85 30-Dec-04 "WORLD TRADE MANAGEMENT M/SSUNSET LANE 1 Main Branch Lahore PB WORLD TRADE MANAGEMENT M/S LCY 2011455219 CURR PKR 75 19-Mar-04 NO.4,PHASE 11 EXTENTION D.H.A KARACHI " "BASFA INDUSTRIES (PVT) LTD.FEROZE PUR 1 Main Branch Lahore PB 0301754-7 BASFA INDUSTRIES (PVT) LTD. -

Sr. No College Name District Gender Division Contact 1 GOVT. COLLEGE for WOMEN, CHUNIAN, KASUR KASUR Female LAHORE 494311934
Sr. College Name District Gender Division Contact No 1 GOVT. COLLEGE FOR WOMEN, CHUNIAN, KASUR KASUR Female LAHORE 494311934 2 GOVT. CHANDO BEGUM DEGREE COLLEGE FOR WOMEN, KANGANPUR, CHUNIAN KASUR Female LAHORE 424820777 3 GOVT. DEGREE COLLEGE FOR WOMEN, ELLAHBAD, DISTRICT KASUR KASUR Female LAHORE 4 GOVT. POST GRAUDATE COLLEGE FOR WOMEN KASUR KASUR Female LAHORE 492771477 5 GOVT. COLLEGE FOR WOMEN KOT RADHA KISHAN, KASUR KASUR Female LAHORE 492385268 Govt. Syed Charagh Shah Degree College for Women, Kot Murad Khan, District 6 KASUR Female LAHORE 492771999 Kasur 7 Govt.Hanifa Begum Degree College For Women Mustafabad,District Kasur KASUR Female LAHORE 8 GOVT. DEGREE COLLEGE FOR WOMEN KHUDIAN KHAS, KASUR KASUR Female LAHORE 492790018 9 GOVT. COLLEGE FOR WOMEN PATTOKI, KASUR KASUR Female LAHORE 494420179 10 GOVT. COLLEGE FOR WOMEN PHOOL NAGAR, KASUR KASUR Female LAHORE 494510398 11 GOVT. COLLEGE (BOYS) CHUNIAN, KASUR KASUR Male LAHORE 494311634 12 Govt. Institute of Commerce, Chunian KASUR Male LAHORE 494311150 13 GOVT. DEGREE COLLEGE MUSTAFA ABAD, KASUR KASUR Male LAHORE 492450518 14 GOVT. DEGREE COLLEGE (B) KOT RADHA KISHAN, KASUR KASUR Male LAHORE 492385525 15 GOVT. ISLAMIA COLLEGE KASUR KASUR Male LAHORE 492727861 16 Govt. College of Commerce, Kasur KASUR Male LAHORE 3049251951 17 GOVT. DEGREE COLLEGE, PATTOKI KASUR Male LAHORE 494420059 18 GOVT. DEGREE COLLEGE BHAI PHERU (PHOOL NAGAR), KASUR KASUR Male LAHORE 494510949 19 Govt. Institute of Commerce, Pattoki KASUR Male LAHORE 494427177 20 GOVT. COLLEGE TOWNSHIP LAHORE LAHORE Both LAHORE 4299262114 21 GOVT. COLLEGE OF SCIENCE WAHDAT ROAD LAHORE LAHORE Both LAHORE 4299260040 22 GOVT. ISLAMIA COLLEGE OF COMMERCE, LAHORE LAHORE Both LAHORE 4237808254 23 GOVT. -

KASUR AGE AS on Marks Marks NEW SR
LIST OF CANDIDATES FOR THE POST OF MALI DISTRICT KASUR AGE AS ON Marks Marks NEW SR. Appl. FATHER DOMICILE EXPERIEN DOCUMEN REMARK Obtained Total FORM NO. NAME GENDER RELEG: CNIC NO. DOB 7 Apr 2021 ADDRESS EDU: MOB NO QUOTA Obtained Merit NO. No. NAME DISTRICT CE T STATUS S in Marks Y M D in Test Interview Jaguwala, 35103- Muhamm Nazir Chak No. 0309- Open Affidavit 1 1 1 Male Islam 0258772- 28-Apr-01 19 11 9 Kasur F.Sc NIL Rejected not ad Navid Ahmad 40, Tehsil 6763433 Merit 5 Pattoki, attatched Kasur 35102- House No. 5% Esperienc Minhas Pervez 09, 0302- e, 2 2 2 Male Christian 6435178- 1-Apr-94 27 0 6 Kasur Middle Miniority NIL Rejected Pervez Masih Christian 4819566 Affidavit 5 Street, Qouta not New Bazar, 35102- P.O. attatchedEsperienc Muhamm Muhammad HussainKasur 0307- Open e, 3 3 3 Male Islam 8049065- 11-Dec-00 20 3 26 Kasur Middle NIL Rejected ad Irshad Aslam Khanuwala 8791836 Merit Affidavit 5 , Baggay, not P.O.Kasur Khas, attatchedEsperienc 35103- Muhamm Rasheed Jaguwala, 0315- Open e, 4 4 4 Male Islam 4993048- 25-Aug-86 34 7 12 Kasur Middle NIL Rejected ad Sufyan Ahmad Chak No. 6365858 Merit Affidavit 5 40, Pattoki, not Kasur attatched 35102- P.O. 5% Nadir Karamat 0304- Affidavit 5 5 5 Male Christian 5081422- 14-Feb-00 21 1 23 Dostpura, Kasur Matric Miniority NIL Rejected not Masigh Masih Fatehpur, 4403534 attatched 3 Kasur Qouta 35102- Nisar Muhammad Village 0304- Open Affidavit 6 6 6 Male Islam 0902556- 15-Mar-87 34 0 22 Sattoki, Kasur Middle NIL Rejected not Ahmad Arshad 4229226 Merit 3 Kasur attatched Muhammad 35102- Affidavit Ali Nizampura, 0307- Open 7 7 7 Mansha Male Islam 1871305- 7-Jan-01 20 3 0 Kasur Matric NIL Rejected not Murtaza Kasur 6867673 Merit Naz 9 attatched 35102- Affidavit Abdul Muhammad Green Kot, 0305- Open 8 8 8 Male Islam 8312169- 5-Feb-97 24 2 2 Kasur Middle NIL Rejected not Rehman Hanif Kasur 5033616 Merit 1 attatched Muhamm 35102- Esperienc Muhammad P.O. -

38456-037: Power Distribution Enhancement Investment Program
Resettlement Plan Due Diligence June 2013 MFF 0021-PAK: Power Distribution Enhancement Investment Program – Tranche 4 Prepared by Lahore Electric Supply Company for the Asian Development Bank. This is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Due Diligence Document Document Stage: Final Project Number: L1-L67 {June 2013} Islamic Republic of Pakistan: Multi-Tranche Financing Facility (MFF) for Power Distribution Enhancement Investment Program Tranche-IV: Power Transformer’s Extension & Augmentation Subprojects Prepared by: Environment & Social Safeguards Section Project Management Unit (PMU) LESCO, Pakistan. i TABLE OF CONTENTS EXECUTIVE SUMMARY .............................................................................................................................................. iv 1 Project Overview ...................................................................................................................................................... 1 1.1 Project Background ........................................................................................................................... 1 2 Scope of Land Acquisition and Resettlement ....................................................................................................... 4 2-1 Scope and Rationale for Land Acquisition ......................................................................................... 4 2-1-1 Site Identification -

Audit Report on the Accounts of Tehsil Municipal Administrations District Kasur
w AUDIT REPORT ON THE ACCOUNTS OF TEHSIL MUNICIPAL ADMINISTRATIONS DISTRICT KASUR AUDIT YEAR 2016-17 AUDITOR GENERAL OF PAKISTAN 1 TABLE OF CONTENTS ABBREVIATIONS AND ACRONYMS ............................................ i PREFACE .......................................................................................... ii EXECUTIVE SUMMARY ............................................................... iii SUMMARY TABLES & CHARTS ................................................. vi Table 1: Audit Work Statistics ........................................................... vi Table 2: Audit observation regarding Financial Management ............. vi Table 3: Outcome Statistics ............................................................... vii Table 4: Irregularities pointed out..................................................... viii Table 5: Cost-Benefit ....................................................................... viii CHAPTER 1 ...................................................................................... 1 1.1 Tehsil Municipal Administrations of District Kasur ............... 1 1.1.1 Introduction ........................................................................... 1 1.1.2 Comments on Budget and Accounts (Variance Analysis) ...... 2 1.1.3 Brief Comments on the Status of Compliance on MFDAC Audit Paras of Audit Report 2015-16 ..................................... 3 1.1.4 Brief Comments on Status of Compliance with PAC Directives ............................................................................. -
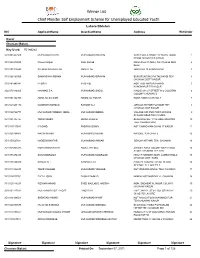
Lahore Division NIC Applicantname Guardianname Address Winorder
Winner List Chief Minister Self Employment Scheme for Unemployed Educated Youth Lahore Division NIC ApplicantName GuardianName Address WinOrder Kasur Chunian (Bolan) Key Used: 761n82x2 3510303447509 MUHAMMAD ILYAS MUHAMMAD IBRAHIM SAFFA WALA CHOKE REHMAN TOWN 1 PHOOL NAGAR DIS KASUR 3510128210995 Naveed Anjum Nazir Ahmad Nizam Pura Ch NO.2, Teh Chunian Distt: 2 Kasur. 3510101725445 SALMAN SANAULLAH ANAULLAH WARD NO 15 KANGAN PUR 3 3510166163569 SHAHNSHAH REHAN MUHAMMAD IBRAHIM BURJ RUN SING PO TALWANDI TEH 4 CHUNIAN DISTT KASUR 3510182983041 M.IQBAL M.ISHAQ MOH HASHIM PURA MANDI 5 KANGNAPUR T/D KASUR 3520170416965 HAMMAD ZIA MUHAMMAD SADIQ H.NO E-241/37 STREET N-2 GULISTAN 6 COLONY CHUNIAN D 3510161322959 ASAD ALI ZAHEER ASHIQ ALI GOHAR ROSA TIBBA CHAK NO. 1 7 3510124934179 NADEEM SHAHZAD RAHMAT ALI GEHLAN HITHAR PO SAME TEH 8 CHUNIAN DIST KASUR 3510124726773 MUHAMMAD TAQEER IQBAL MUHAMMAD IQBBAL VILLAGE GID PUR POST OFFOCE 9 SHAMAS ABAD TEH CHUNIA 3510191707727 TARIQ SAEED ABDUL KHALIQ BHAGIWAL NO. 1 P/O GEHLAN HITAR 10 TEH. CHUNIAN DIST. 3510111317093 M ASHIQ FAQIR HUSSAIN KOT CHAND KAN CH NO 17 KASUR 11 3510121188807 WASIM MUNIR MUHAMMSD MUNIR RASOOL PUR CHAK 5 12 3510155627681 NADEEM AKHTAR MUHAMMAD ANWAR GEHLAN HITHAR, TEH. CHUNIAN 13 3510135965485 MOHAMMAD IKRAM ABDUL HAFEEZ ZAHEER ABAD COLONY NEAR COLD 14 STORE CHUNIAN TEH CHU 3510178274493 ZAIN MAQSOOD MUHAMMAD MAQSOOD H#1217 WARD#16 MOH. LAMBA KHOLA 15 CHUNIAN DIST. KASU 3510178698919 SHAKIR ALI HASHMAT ALI H NO 29-1 MUHALLAH NIL BLOCK 16 SECTOR -D -II GREEN T 3510143322673 TAHIR YAQOOB MUHAMMAD YAQOOB KOT WASAWA SINGH, TEH. -
QUARTERLY ACTIVITY REPORT April-June 2010
QUARTERLY ACTIVITY REPORT April-June 2010 By Human and Institutional Development Section 26-C. Nawab Town, Raiwind Road, Lahore PHONE: +92-42-5310471-2, FAX: +92-42-5310473, www.damen-pk.org,E-mail: [email protected] OVERVIEW During the quarter April-June 2010 DAMEN has laid strong emphasis to build up as a strong institution for delivering economic and social services to the people of the marginalized communities. DAMEN has always maintained the stance that economic opportunities along with social sector initiatives are the best solution to poverty eradication. The merger of economic and social activities has created a strong model for combating poverty in the operational areas of DAMEN and will be successful in other areas in future. One of the most important aspects of DAMEN’s endeavors is the blending of social and economic initiatives into a new integrated model of poverty alleviation. The field offices are not only extending their financial services to the clients but also taking a proactive role in the provision of educational and health facilities to the people of their communities. This new approach will provide a new dimension to DAMEN’s efforts to bring a sustainable change in the social and economic status of the people in the target communities. Presently DAMEN is operating through 2 area offices at district Lahore along with 10 field offices, 1 area office and 5 field offices at district Sheikhupura and 1 area office and 5 field offices at district Kasur along with 100 home schools and 15 health centers. All the field offices are not only operationally sustainable but also covering all cost of the social sector program of DAMEN and supporting area office as well.