Nematoda: Cosmocercidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Froglog95 New Version Draft1.Indd
March 2011 Vol. 95 FrogLogwww.amphibians.org News from the herpetological community The new face of the ASG “Lost” Frogs Red List The global search Updating South comes to an end. Africas Red Where next? Lists. Page 1 FrogLog Vol. 95 | March 2011 | 1 2 | FrogLog Vol. 95 | March 2011 CONTENTS The Sierra Caral of Guatemala a refuge for endemic amphibians page 5 The Search for “Lost” Frogs page 12 Recent diversifi cation in old habitats: Molecules and morphology in the endangered frog, Craugastor uno page 17 Updating the IUCN Red List status of South African amphibians 6 Amphibians on the IUCN Red List: Developments and changes since the Global Amphibian Assessment 7 The forced closure of conservation work on Seychelles Sooglossidae 8 Alien amphibians challenge Darwin’s naturalization hypothesis 9 Is there a decline of amphibian richness in Bellanwila-Attidiya Sanctuary? 10 High prevalence of the amphibian chytrid pathogen in Gabon 11 Breeding-site selection by red-belly toads, Melanophryniscus stelzneri (Anura: Bufonidae), in Sierras of Córdoba, Argentina 11 Upcoming meetings 20 | Recent Publications 20 | Internships & Jobs 23 Funding Opportunities 22 | Author Instructions 24 | Current Authors 25 FrogLog Vol. 95 | March 2011 | 3 FrogLog Editorial elcome to the new-look FrogLog. It has been a busy few months Wfor the ASG! We have redesigned the look and feel of FrogLog ASG & EDITORIAL COMMITTEE along with our other media tools to better serve the needs of the ASG community. We hope that FrogLog will become a regular addition to James P. Collins your reading and a platform for sharing research, conservation stories, events, and opportunities. -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -

Rediscovery at the Type Locality and Detection of a New Population 1José L
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [General Section]: 126–132 (e193). Thorius narismagnus (Amphibia: Plethodontidae): rediscovery at the type locality and detection of a new population 1José L. Aguilar-López, 2,*Paulina García-Bañuelos, 3Eduardo Pineda, and 4Sean M. Rovito 1,2,3Red de Biología y Conservación de Vertebrados, Instituto de Ecología, A.C., Carretera antigua a Coatepec 351, El Haya, Xalapa, Veracruz, MEXICO 4Unidad de Genómica Avanzada (Langebio), Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, km 9.6 Libramiento Norte Carretera Irapuato-León, Irapuato, Guanajuato CP 36824, MEXICO Abstract.—Of the 42 Critically Endangered species of plethodontid salamanders that occur in Mexico, thirteen have not been reported in more than ten years. Given the lack of reports since 1976, the minute plethodontid salamander Thorius narismagnus is widely considered as missing. However, this report describes the rediscovery of this minute salamander at the type locality (Volcán San Martín), as well as a new locality on Volcán Santa Marta, 28 km southeast of its previously known distribution, both in the Los Tuxtlas region of Veracruz, Mexico. The localities where T. narismagnus has been found are mature forests in a community reserve on Volcán San Martín and a private reserve on Volcán Santa Marta. The presence of maxillary teeth, generally absent in Thorius, are reported here in some T. narismagnus females. Two efforts which may contribute to the conservation of Thorius narismagnus are the preservation of the cloud forests where this species persists, as well as the determination of the presence and possible effect of chytrid fungus in these populations. -

Osteological Variation Among Extreme
Central Washington University ScholarWorks@CWU All Faculty Scholarship for the College of the Sciences College of the Sciences 6-10-2015 Osteological Variation among Extreme Morphological Forms in the Mexican Salamander Genus Chiropterotriton (Amphibia: Plethodontidae): Morphological Evolution And Homoplasy David M. Darda David B. Wake Follow this and additional works at: https://digitalcommons.cwu.edu/cotsfac Part of the Ecology and Evolutionary Biology Commons RESEARCH ARTICLE Osteological Variation among Extreme Morphological Forms in the Mexican Salamander Genus Chiropterotriton (Amphibia: Plethodontidae): Morphological Evolution And Homoplasy David M. Darda1*, David B. Wake2 1 Department of Biological Sciences, Central Washington University, Ellensburg, Washington, United States of America, 2 Department of Integrative Biology and Museum of Vertebrate Zoology, University of California, Berkeley, California, United States of America * [email protected] Abstract Osteological variation is recorded among and within four of the most distinctive species of the Mexican salamander genus Chiropterotriton. Analysis of the data is consistent with the OPEN ACCESS monophyletic status of the genus and documents previously unrecorded intraspecific and Citation: Darda DM, Wake DB (2015) Osteological interspecific variation. Most of the recorded variation involves qualitative and quantitative Variation among Extreme Morphological Forms in the proportional differences, but four fixed differences constitute autapomorphic states that af- Mexican Salamander Genus Chiropterotriton firm and diagnose some species (C. dimidiatus, C. magnipes). Osteological variation in 15 (Amphibia: Plethodontidae): Morphological Evolution characters is analyzed with respect to predictions generated from four hypotheses: 1) phy- And Homoplasy. PLoS ONE 10(6): e0127248. doi:10.1371/journal.pone.0127248 logeny, 2) adaptation to specific habitats (the four species include cave-dwelling, terrestrial, and arboreal forms), 3) size-free shape, and 4) size. -
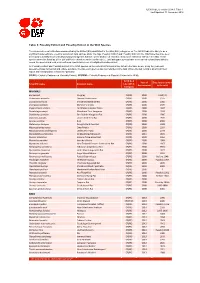
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2014.3: Table 9 Last Updated: 13 November 2014 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific name Common name List (2014) Assessment in the wild Category MAMMALS Bos sauveli Kouprey CR(PE) 2008 1969/70 Crateromys australis Dinagat Crateromys CR(PE) 2008 1975 Crocidura trichura Christmas Island Shrew CR(PE) 2008 1985 Crocidura wimmeri -

Amphibian Diversity and Threatened Species in a Severely Transformed Neotropical Region in Mexico
RESEARCH ARTICLE Amphibian Diversity and Threatened Species in a Severely Transformed Neotropical Region in Mexico Yocoyani Meza-Parral, Eduardo Pineda* Red de Biología y Conservación de Vertebrados, Instituto de Ecología, A.C., Xalapa, Veracruz, México * [email protected] Abstract Many regions around the world concentrate a large number of highly endangered species that have very restricted distributions. The mountainous region of central Veracruz, Mexico, is considered a priority area for amphibian conservation because of its high level of ende- mism and the number of threatened species. The original tropical montane cloud forest in OPEN ACCESS the region has been dramatically reduced and fragmented and is now mainly confined to ra- Citation: Meza-Parral Y, Pineda E (2015) Amphibian vines and hillsides. We evaluated the current situation of amphibian diversity in the cloud Diversity and Threatened Species in a Severely forest fragments of this region by analyzing species richness and abundance, comparing Transformed Neotropical Region in Mexico. PLoS assemblage structure and species composition, examining the distribution and abundance ONE 10(3): e0121652. doi:10.1371/journal. pone.0121652 of threatened species, and identifying the local and landscape variables associated with the observed amphibian diversity. From June to October 2012 we sampled ten forest frag- Academic Editor: Stefan Lötters, Trier University, GERMANY ments, investing 944 person-hours of sampling effort. A total of 895 amphibians belonging to 16 species were recorded. Notable differences in species richness, abundance, and as- Received: May 22, 2014 semblage structure between forest fragments were observed. Species composition be- Accepted: November 11, 2014 tween pairs of fragments differed by an average of 53%, with the majority (58%) resulting Published: March 23, 2015 from species replacement and the rest (42%) explained by differences in species richness. -

Results of the Global Amphibian Assessment for Bolivia
Results of the Global Amphibian Assessment for Bolivia Diversity Number of Rank in Latin World Rank2 Percentage of Species in Bolivia America and the the World’s Caribbean1 Diversity All Amphibians 201 7 15 3.5 % Frogs & Toads 197 7 14 3.9 % Salamanders 1 13 68 0.2 % Caecilians 3 11 20 1.8 % 1 Out of 44 countries and territories. 2 Out of 192 countries and territories. Threatened Species (Threatened species are in one of the categories in italics.) IUCN Categories Number of Species in IUCN Categories Number of Species in Bolivia Bolivia Extinct 0 Near Threatened 6 Critically Endangered 5 Least Concern 161 Endangered 6 Data Deficient 13 Vulnerable 10 Number and percent of Bolivian species that are threatened: 21 (10%) Extinct Species: none Species that are Critically Endangered and possibly extinct (species that are missing but for which sufficient information is lacking to declare them as extinct): Frogs: Gastrotheca lauzuricae, Hyla chlorostea, Eleutherodactylus zongoensis Role of Protected Areas: Of the 21 threatened species in Bolivia, 76% occur in at least one protected area. Local Experts: Steffen Reichle, The Nature Conservancy, TEL (591-3) 348 0766, 348 0767 Claudia Cortez, Colección Boliviana de Fauna, TEL (591-3) 272 1152 For More Information: Dr. Bruce Young, NatureServe, Costa Rica, TEL (506) 645-6231 Global Amphibian Assessment website: www.globalamphibians.org A detailed analysis of the results for the Americas: www.natureserve.org Results of the Global Amphibian Assessment for Bolivia Map of Amphibian Diversity Source: Global Amphibian Assessment Map of the Distribution of Threatened Species Source: Global Amphibian Assessment Results of the Global Amphibian Assessment for Brazil Diversity Number of Rank in Latin World Rank2 Percentage of Species in Brazil America and the the World’s Caribbean1 Diversity All Amphibians 731 1 1 12.7 % Frogs & Toads 704 1 1 13.9 % Salamanders 1 13 68 0.2 % Caecilians 26 2 2 15.5 % 1 Out of 44 countries and territories. -

Minelli-Et-Al(Eds)
ZOOTAXA 1950 Updating the Linnaean Heritage: Names as Tools for Thinking about Animals and Plants ALESSANDRO MINELLI, LUCIO BONATO & GIUSEPPE FUSCO (EDS) Magnolia Press Auckland, New Zealand ALESSANDRO MINELLI, LUCIO BONATO & GIUSEPPE FUSCO (EDS) Updating the Linnaean Heritage: Names as Tools for Thinking about Animals and Plants (Zootaxa 1950) 163 pp.; 30 cm. 5 Dec. 2008 ISBN 978-1-86977-297-0 (paperback) ISBN 978-1-86977-298-7 (Online edition) FIRST PUBLISHED IN 2008 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2008 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) Zootaxa 1950: 3–4 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Updating the Linnaean Heritage: Names as Tools for Thinking about Animals and Plants ALESSANDRO MINELLI FLS, LUCIO BONATO & GIUSEPPE FUSCO (EDS) Department of Biology, University of Padova, Via Ugo Bassi 58B, I 35131 Padova, Italy Email: [email protected], [email protected], [email protected] Table of contents 4 Preface ALESSANDRO MINELLI FLS, LUCIO BONATO, GIUSEPPE FUSCO (ITALY) 5 Actual usage of biological nomenclature and its implications for data integrators; a national, regional and global perspective CHARLES HUSSEY (UK), YDE DE JONG (THE NETHERLANDS), DAVID REMSEN (DENMARK) 9 The Linnean foundations of zoological and botanical nomenclature OTTO KRAUS (GERMANY) 21 Zoological vs. -
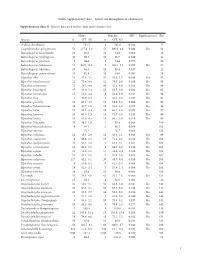
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Genome Size Diversification in Central American Bolitoglossine Salamanders (Caudata; Plethodontidae)
Copeia 107, No. 3, 2019, 560–566 Genome Size Diversification in Central American Bolitoglossine Salamanders (Caudata; Plethodontidae) Michael W. Itgen1,PatrikPrˇsa2,RokJanˇza3, Lucijan Skubic4,JosiahH.Townsend5,Aleˇs Kladnik6, Lilijana Bizjak Mali6, and Stanley K. Sessions7 Genome sizes (expressed as C-values, or haploid genome sizes) of six species of Honduran plethodontid salamanders (one species of Nototriton and five of Bolitoglossa) vary greatly. Nototriton has a moderate-sized genome (29.2 pg) relative to other species of salamanders. Genome sizes in the species of Bolitoglossa span a range of 24 pg (~23.4 gigabases) of DNA and include the largest genomes (83.7 pg) reported for the genus and for the family Plethodontidae. A phylogenetic analysis indicates that genome evolution in this group of salamanders featured mainly large increases in the mass of nuclear DNA. We propose that these evolutionary changes in genome size reflect random drift in small, isolated populations in the highlands of Central America. ALAMANDERS show more variation in genome size whole organismal level (Sessions and Larson, 1987; Roth et than any other vertebrate order (Gregory, 2005). Mean al., 1994; Gregory, 2005). Both nuclear volume and overall S C-values in salamanders range from 13.8 picograms cell size are positively correlated with genome size, and the (pg) to over 120 pg of DNA (Gregory, 2016). This genome size combination of large cell and small body size can lead to variation does not involve polyploidy, and instead is driven developmental constraints that have probably played an by transposable elements (TEs) and other non-coding DNA important role in the morphological evolution of pletho- sequences (such as repetitive sequences) spread across the dontid salamanders (Sessions, 1985; Sessions and Larson, karyotype, affecting chromosome size but not number or 1987; Hanken and Wake, 1993; Roth et al., 1994, 1997; Parra- shape between species (Mizuno and Macgregor, 1974; Orgel Olea et al., 2004; Sessions, 2008).