Page 1 of 23 RSC Advances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vegetation, Floristic Composition and Species Diversity in a Tropical Mountain Nature Reserve in Southern Yunnan, SW China, with Implications for Conservation
Mongabay.com Open Access Journal - Tropical Conservation Science Vol.8 (2): 528-546, 2015 Research Article Vegetation, floristic composition and species diversity in a tropical mountain nature reserve in southern Yunnan, SW China, with implications for conservation Hua Zhu*, Chai Yong, Shisun Zhou, Hong Wang and Lichun Yan Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Xue-Fu Road 88, Kunming, Yunnan 650223, P. R. China Tel.: 0086-871-65171169; Fax: 0086-871-65160916 *Corresponding author: H. Zhu, e-mail [email protected]; Fax no.: 86-871-5160916 Abstract Complete floristic and vegetation surveys were done in a newly established nature reserve on a tropical mountain in southern Yunnan. Three vegetation types in three altitudinal zones were recognized: a tropical seasonal rain forest below 1,100 m; a lower montane evergreen broad- leaved forest at 1,100-1,600 m; and a montane rain forest above 1,600 m. A total of 1,657 species of seed plants in 758 genera and 146 families were recorded from the nature reserve. Tropical families (61%) and genera (81%) comprise the majority of the flora, and tropical Asian genera make up the highest percentage, showing the close affinity of the flora with the tropical Asian (Indo-Malaysia) flora, despite the high latitude (22N). Floristic changes with altitude are conspicuous. The transition from lowland tropical seasonal rain forest dominated by mixed tropical families to lower montane forest dominated by Fagaceae and Lauraceae occurs at 1,100-1,150 m. Although the middle montane forests above 1,600 m have ‘oak-laurel’ assemblage characteristics, the temperate families Magnoliaceae and Cornaceae become dominant. -

Ultramafic Geocology of South and Southeast Asia
Galey et al. Bot Stud (2017) 58:18 DOI 10.1186/s40529-017-0167-9 REVIEW Open Access Ultramafc geoecology of South and Southeast Asia M. L. Galey1, A. van der Ent2,3, M. C. M. Iqbal4 and N. Rajakaruna5,6* Abstract Globally, ultramafc outcrops are renowned for hosting foras with high levels of endemism, including plants with specialised adaptations such as nickel or manganese hyperaccumulation. Soils derived from ultramafc regoliths are generally nutrient-defcient, have major cation imbalances, and have concomitant high concentrations of potentially phytotoxic trace elements, especially nickel. The South and Southeast Asian region has the largest surface occur- rences of ultramafc regoliths in the world, but the geoecology of these outcrops is still poorly studied despite severe conservation threats. Due to the paucity of systematic plant collections in many areas and the lack of georeferenced herbarium records and databased information, it is not possible to determine the distribution of species, levels of end- emism, and the species most threatened. However, site-specifc studies provide insights to the ultramafc geoecology of several locations in South and Southeast Asia. The geoecology of tropical ultramafc regions difers substantially from those in temperate regions in that the vegetation at lower elevations is generally tall forest with relatively low levels of endemism. On ultramafc mountaintops, where the combined forces of edaphic and climatic factors inter- sect, obligate ultramafc species and hyperendemics often occur. Forest clearing, agricultural development, mining, and climate change-related stressors have contributed to rapid and unprecedented loss of ultramafc-associated habitats in the region. The geoecology of the large ultramafc outcrops of Indonesia’s Sulawesi, Obi and Halmahera, and many other smaller outcrops in South and Southeast Asia, remains largely unexplored, and should be prioritised for study and conservation. -

Acanthaceae), a New Chinese Endemic Genus Segregated from Justicia (Acanthaceae)
Plant Diversity xxx (2016) 1e10 Contents lists available at ScienceDirect Plant Diversity journal homepage: http://www.keaipublishing.com/en/journals/plant-diversity/ http://journal.kib.ac.cn Wuacanthus (Acanthaceae), a new Chinese endemic genus segregated from Justicia (Acanthaceae) * Yunfei Deng a, , Chunming Gao b, Nianhe Xia a, Hua Peng c a Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, People's Republic of China b Shandong Provincial Engineering and Technology Research Center for Wild Plant Resources Development and Application of Yellow River Delta, Facultyof Life Science, Binzhou University, Binzhou, 256603, Shandong, People's Republic of China c Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, People's Republic of China article info abstract Article history: A new genus, Wuacanthus Y.F. Deng, N.H. Xia & H. Peng (Acanthaceae), is described from the Hengduan Received 30 September 2016 Mountains, China. Wuacanthus is based on Wuacanthus microdontus (W.W.Sm.) Y.F. Deng, N.H. Xia & H. Received in revised form Peng, originally published in Justicia and then moved to Mananthes. The new genus is characterized by its 25 November 2016 shrub habit, strongly 2-lipped corolla, the 2-lobed upper lip, 3-lobed lower lip, 2 stamens, bithecous Accepted 25 November 2016 anthers, parallel thecae with two spurs at the base, 2 ovules in each locule, and the 4-seeded capsule. Available online xxx Phylogenetic analyses show that the new genus belongs to the Pseuderanthemum lineage in tribe Justi- cieae. -

Hypoestes Aristata (Vahl) Sol
Biol Res 43: 403-409, 2010 BHATT ET AL. Biol Res 43, 2010, 403-409 B403R The foliar trichomes of Hypoestes aristata (Vahl) Sol. ex Roem. & Schult var aristata (Acanthaceae) a widespread medicinal plant species in tropical sub-Saharan Africa: with comments on its possible phylogenetic significance A. Bhatt*, Y. Naidoo and A. Nicholas School of Biological and Conservation Sciences, University of KwaZulu-Natal, Westville Campus, Private Bag X54001, Durban, KZN, 4000, South Africa ABSTRACT The micromorphology of foliar trichomes of Hypoestes aristata var. aristata was studied using stereo, light and scanning microscopy (SEM). This genus belongs to the advanced angiosperm family Acanthaceae, for which few micromorphological leaf studies exist. Results revealed both glandular and non-glandular trichomes, the latter being more abundant on leaf veins, particularly on the abaxial surface of very young leaves. With leaf maturity, the density of non-glandular trichomes decreased. Glandular trichomes were rare and of two types: long-stalked capitate and globose-like peltate trichomes. Capitate trichomes were observed only on the abaxial leaf surface, while peltate trichomes were distributed on both adaxial and abaxial leaf surfaces. Key terms: Acanthaceae, Glandular trichomes, Hypoestes aristata var. aristata, medicinal plant, Scanning electron microscope. INTRODUCTION zygomorphic flowers supported by prominent bracts and producing explosive capsular fruits. Many studies have The Family Acanthaceae is a large and diverse family of further supported the placement of Hypoestes in a smaller dicotyledonous plants comprising about 202 genera and 3520 clade that includes the prominent genus Justicia (McDade species (Judd et al., 2008); although estimates vary from 2600 and Moody 1999). -

Check List of Wild Angiosperms of Bhagwan Mahavir (Molem
Check List 9(2): 186–207, 2013 © 2013 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Check List of Wild Angiosperms of Bhagwan Mahavir PECIES S OF Mandar Nilkanth Datar 1* and P. Lakshminarasimhan 2 ISTS L (Molem) National Park, Goa, India *1 CorrespondingAgharkar Research author Institute, E-mail: G. [email protected] G. Agarkar Road, Pune - 411 004. Maharashtra, India. 2 Central National Herbarium, Botanical Survey of India, P. O. Botanic Garden, Howrah - 711 103. West Bengal, India. Abstract: Bhagwan Mahavir (Molem) National Park, the only National park in Goa, was evaluated for it’s diversity of Angiosperms. A total number of 721 wild species belonging to 119 families were documented from this protected area of which 126 are endemics. A checklist of these species is provided here. Introduction in the National Park are Laterite and Deccan trap Basalt Protected areas are most important in many ways for (Naik, 1995). Soil in most places of the National Park area conservation of biodiversity. Worldwide there are 102,102 is laterite of high and low level type formed by natural Protected Areas covering 18.8 million km2 metamorphosis and degradation of undulation rocks. network of 660 Protected Areas including 99 National Minerals like bauxite, iron and manganese are obtained Parks, 514 Wildlife Sanctuaries, 43 Conservation. India Reserves has a from these soils. The general climate of the area is tropical and 4 Community Reserves covering a total of 158,373 km2 with high percentage of humidity throughout the year. -

Download This Article As
Int. J. Curr. Res. Biosci. Plant Biol. (2019) 6(10), 33-46 International Journal of Current Research in Biosciences and Plant Biology Volume 6 ● Number 10 (October-2019) ● ISSN: 2349-8080 (Online) Journal homepage: www.ijcrbp.com Original Research Article doi: https://doi.org/10.20546/ijcrbp.2019.610.004 Some new combinations and new names for Flora of India R. Kottaimuthu1*, M. Jothi Basu2 and N. Karmegam3 1Department of Botany, Alagappa University, Karaikudi-630 003, Tamil Nadu, India 2Department of Botany (DDE), Alagappa University, Karaikudi-630 003, Tamil Nadu, India 3Department of Botany, Government Arts College (Autonomous), Salem-636 007, Tamil Nadu, India *Corresponding author; e-mail: [email protected] Article Info ABSTRACT Date of Acceptance: During the verification of nomenclature in connection with the preparation of 17 August 2019 ‗Supplement to Florae Indicae Enumeratio‘ and ‗Flora of Tamil Nadu‘, the authors came across a number of names that need to be updated in accordance with the Date of Publication: changing generic concepts. Accordingly the required new names and new combinations 06 October 2019 are proposed here for the 50 taxa belonging to 17 families. Keywords Combination novum Indian flora Nomen novum Tamil Nadu Introduction Taxonomic treatment India is the seventh largest country in the world, ACANTHACEAE and is home to 18,948 species of flowering plants (Karthikeyan, 2018), of which 4,303 taxa are Andrographis longipedunculata (Sreem.) endemic (Singh et al., 2015). During the L.H.Cramer ex Gnanasek. & Kottaim., comb. nov. preparation of ‗Supplement to Florae Indicae Enumeratio‘ and ‗Flora of Tamil Nadu‘, we came Basionym: Neesiella longipedunculata Sreem. -

Acanthaceae and Asteraceae Family Plants Used by Folk Medicinal Practitioners for Treatment of Malaria in Chittagong and Sylhet Divisions of Bangladesh
146 American-Eurasian Journal of Sustainable Agriculture, 6(3): 146-152, 2012 ISSN 1995-0748 ORIGINAL ARTICLE Acanthaceae and Asteraceae family plants used by folk medicinal practitioners for treatment of malaria in Chittagong and Sylhet Divisions of Bangladesh Md. Tabibul Islam, Protiva Rani Das, Mohammad Humayun Kabir, Shakila Akter, Zubaida Khatun, Md. Megbahul Haque, Md. Saiful Islam Roney, Rownak Jahan, Mohammed Rahmatullah Faculty of Life Sciences, University of Development Alternative, Dhanmondi, Dhaka-1205, Bangladesh Md. Tabibul Islam, Protiva Rani Das, Mohammad Humayun Kabir, Shakila Akter, Zubaida Khatun, Md. Megbahul Haque, Md. Saiful Islam Roney, Rownak Jahan, Mohammed Rahmatullah: Acanthaceae and Asteraceae family plants used by folk medicinal practitioners for treatment of malaria in Chittagong and Sylhet Divisions of Bangladesh ABSTRACT Malaria is a debilitating disease causing high mortality rates among men and women if not treated properly. The disease is prevalent in many countries of the world with the most prevalence noted among the sub-Saharan countries, where it is in an epidemic form. The disease is classified as hypo-endemic in Bangladesh with the southeast and the northeastern regions of the country having the most malaria-affected people. The rural people suffer most from malaria, and they rely on folk medicinal practitioners for treatment, who administer various plant species for treatment of the disease as well as associated symptoms like pain and fever. Plant species have always formed the richest sources of anti-malarial drugs, the most notable being quinine and artemisinin. However, quinine has developed drug-resistant vectors and artemisinin is considered by some to developing initial resistance, particularly in China, where it has been used for thousands of years to combat malaria. -

Compositions and Comparison of the Leaf and Stem Essential Oils from Nigerian Hypoestes Phyllostachya ‘ ’ Rosea P
id619187 pdfMachine by Broadgun Software - a great PDF writer! - a great PDF creator! - http://www.pdfmachine.com http://www.broadgun.com September 2009 Volume 5 Issue 3 NNaattuurraall PPrrAoon dIdnduuian ccJotutrnssal Trade Science Inc. Full Paper NPAIJ, 5(3), 2009 [116-119] Compositions and comparison of the leaf and stem essential oils from Nigerian Hypoestes phyllostachya ‘ ’ rosea p. Beau. [Acanthaceae] Dorcas Olufunke Moronkola*, Odunayo Christy Atewolara- Odule, Oseyemi Omowunmi Olubomehin Department of Chemical Sciences, Olabisi Onabanjo University, P.M.B. 2002, Ago-Iwoye, Ogun-State, (NIGERIA) E-mail : [email protected] Received: 22nd May, 2009 ; Accepted: 2nd June, 2009 ABSTRACT KEYWORDS Leaf and stem volatile oils were obtained differently from Hypoestes Hypoestes phyllostachya ‘ ’ [HR] [Acanthaceae] in 0.36% and 0.13% respectively. ‘ ’ phyllostachya Rosea Rosea ; Fourteen main volatiles were identified in the stem part which had Acanthaceae; appreciable amount of sesquiterpenes with the most abundant compounds Naphthalenones; being 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6-(1-methylethenyl)-2(1H) Cyclohexanes; naphthalenone (38.01%), 1-ethenyl-1-methyl-2(1-methylethenyl)-4-(1- Taxonomic compounds; methylethylidene) cyclohexane (14.26%) and tetramethylcyclopropylidene Hydrodistillation. methylbenzene(7.38%). Twenty-five compounds were identified in the leaf oil of HR which also contained appreciable amount of sesquiterpenes. The most abundant compounds were 3,5,6,7,8,8a-hexahydro-4,8a-dimethyl-6- (1-methylethenyl)-2(1H)naphthalenone(23.3%), and1-ethenyl-1-methyl-2- (1-methylethenyl)-4-(1-methylethylidene) cyclohexane(20.73%), which were the same as in the stem parts. The two most abundant compounds are common to both leaf and stem essential oils. -

ACANTHACEAE 爵床科 Jue Chuang Ke Hu Jiaqi (胡嘉琪 Hu Chia-Chi)1, Deng Yunfei (邓云飞)2; John R
ACANTHACEAE 爵床科 jue chuang ke Hu Jiaqi (胡嘉琪 Hu Chia-chi)1, Deng Yunfei (邓云飞)2; John R. I. Wood3, Thomas F. Daniel4 Prostrate, erect, or rarely climbing herbs (annual or perennial), subshrubs, shrubs, or rarely small trees, usually with cystoliths (except in following Chinese genera: Acanthus, Blepharis, Nelsonia, Ophiorrhiziphyllon, Staurogyne, and Thunbergia), isophyllous (leaf pairs of equal size at each node) or anisophyllous (leaf pairs of unequal size at each node). Branches decussate, terete to angular in cross-section, nodes often swollen, sometimes spinose with spines derived from reduced leaves, bracts, and/or bracteoles. Stipules absent. Leaves opposite [rarely alternate or whorled]; leaf blade margin entire, sinuate, crenate, dentate, or rarely pinnatifid. Inflo- rescences terminal or axillary spikes, racemes, panicles, or dense clusters, rarely of solitary flowers; bracts 1 per flower or dichasial cluster, large and brightly colored or minute and green, sometimes becoming spinose; bracteoles present or rarely absent, usually 2 per flower. Flowers sessile or pedicellate, bisexual, zygomorphic to subactinomorphic. Calyx synsepalous (at least basally), usually 4- or 5-lobed, rarely (Thunbergia) reduced to an entire cupular ring or 10–20-lobed. Corolla sympetalous, sometimes resupinate 180º by twisting of corolla tube; tube cylindric or funnelform; limb subactinomorphic (i.e., subequally 5-lobed) or zygomorphic (either 2- lipped with upper lip subentire to 2-lobed and lower lip 3-lobed, or rarely 1-lipped with 3 lobes); lobes ascending or descending cochlear, quincuncial, contorted, or open in bud. Stamens epipetalous, included in or exserted from corolla tube, 2 or 4 and didyna- mous; filaments distinct, connate in pairs, or monadelphous basally via a sheath (Strobilanthes); anthers with 1 or 2 thecae; thecae parallel to perpendicular, equally inserted to superposed, spherical to linear, base muticous or spurred, usually longitudinally dehis- cent; staminodes 0–3, consisting of minute projections or sterile filaments. -

Functional Characterisation of the Transcriptome from Leaf Tissue of The
www.nature.com/scientificreports OPEN Functional characterisation of the transcriptome from leaf tissue of the fuoroacetate‑producing plant, Dichapetalum cymosum, in response to mechanical wounding Selisha A. Sooklal1,4, Phelelani T. Mpangase2,4, Mihai‑Silviu Tomescu1, Shaun Aron2, Scott Hazelhurst2, Robert H. Archer3 & Karl Rumbold1* Dichapetalum cymosum produces the toxic fuorinated metabolite, fuoroacetate, presumably as a defence mechanism. Given the rarity of fuorinated metabolites in nature, the biosynthetic origin and function of fuoroacetate have been of particular interest. However, the mechanism for fuorination in D. cymosum was never elucidated. More importantly, there is a severe lack in knowledge on a genetic level for fuorometabolite‑producing plants, impeding research on the subject. Here, we report on the frst transcriptome for D. cymosum and investigate the wound response for insights into fuorometabolite production. Mechanical wounding studies were performed and libraries of the unwounded (control) and wounded (30 and 60 min post wounding) plant were sequenced using the Illumina HiSeq platform. A combined reference assembly generated 77,845 transcripts. Using the SwissProt, TrEMBL, GO, eggNOG, KEGG, Pfam, EC and PlantTFDB databases, a 69% annotation rate was achieved. Diferential expression analysis revealed the regulation of 364 genes in response to wounding. The wound responses in D. cymosum included key mechanisms relating to signalling cascades, phytohormone regulation, transcription factors and defence‑related secondary metabolites. However, the role of fuoroacetate in inducible wound responses remains unclear. Bacterial fuorinases were searched against the D. cymosum transcriptome but transcripts with homology were not detected suggesting the presence of a potentially diferent fuorinating enzyme in plants. Nevertheless, the transcriptome produced in this study signifcantly increases genetic resources available for D. -

First Steps Towards a Floral Structural Characterization of the Major Rosid Subclades
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2006 First steps towards a floral structural characterization of the major rosid subclades Endress, P K ; Matthews, M L Abstract: A survey of our own comparative studies on several larger clades of rosids and over 1400 original publications on rosid flowers shows that floral structural features support to various degrees the supraordinal relationships in rosids proposed by molecular phylogenetic studies. However, as many apparent relationships are not yet well resolved, the structural support also remains tentative. Some of the features that turned out to be of interest in the present study had not previously been considered in earlier supraordinal studies. The strongest floral structural support is for malvids (Brassicales, Malvales, Sapindales), which reflects the strong support of phylogenetic analyses. Somewhat less structurally supported are the COM (Celastrales, Oxalidales, Malpighiales) and the nitrogen-fixing (Cucurbitales, Fagales, Fabales, Rosales) clades of fabids, which are both also only weakly supported in phylogenetic analyses. The sister pairs, Cucurbitales plus Fagales, and Malvales plus Sapindales, are structurally only weakly supported, and for the entire fabids there is no clear support by the present floral structural data. However, an additional grouping, the COM clade plus malvids, shares some interesting features but does not appear as a clade in phylogenetic analyses. Thus it appears that the deepest split within eurosids- that between fabids and malvids - in molecular phylogenetic analyses (however weakly supported) is not matched by the present structural data. Features of ovules including thickness of integuments, thickness of nucellus, and degree of ovular curvature, appear to be especially interesting for higher level relationships and should be further explored. -
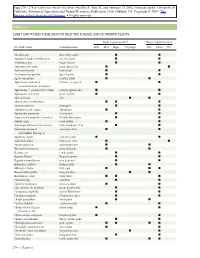
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum