Functional Characteristics Analysis of Dehydrins in Larix Kaempferi Under Osmotic Stress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tsdhn-2, a Unique Dehydrin Protein from Thellungiella and Its Role in Salt Tolerance
TsDHN-2, A Unique Dehydrin Protein from Thellungiella and its Role in Salt Tolerance A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Department of Biochemistry University of Saskatchewan Saskatoon by Sarah Catherine Klatt © Copyright Sarah C. Klatt, July, 2011. All rights reserved. - i - PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a master’s degree from the University of Saskatchewan. I agree that the Libraries of this University may make it freely available for inspection. Moreover, I agree that permission for copying of this thesis in any manner, in whole or in part, for scholarly purposes may be granted by the department or the dean of the college in which this thesis work was done. It is understood that any copying or publication or use of this thesis or parts there of for financial gain without approval by the University of Saskatchewan and the author’s written permission is prohibited. It is also understood that due recognition shall be given to the author and to the University of Saskatchewan in any scholarly use which may be made of any material used in this thesis. Requests for permission to copy or to make either use of the material presented in this thesis in while or part should be addressed to: Head of the Department of Biochemistry University of Saskatchewan Saskatoon, Saskatchewan S7N 5A8 CANADA - iii - - ABSTRACT Salt stress, or salinity, is one of the most common environmental stresses affecting crop yield worldwide. -

Invasive Alien Species Fact Sheet Phytophthora Ramorum
NOBANIS –Invasive Alien Species Fact Sheet Phytophthora ramorum Author of this species fact sheet: Anna Poimala and Arja Lilja, Finnish Forest Research Institute, Vantaa Research Unit, PO Box 18, 01301 Vantaa, Finland; +358 40 801 5377 ; [email protected] Bibliographical reference – how to cite this fact sheet: Poimala, A. & Lilja, A. (2013): NOBANIS – Invasive Alien Species Fact Sheet – Phytophthora ramorum . – From: Online Database of the European Network on Invasive Alien Species – NOBANIS www.nobanis.org , Date of access x/x/201x. Species description Scientific names: Phytophthora ramorum Werres, De Cock & Man in`t Veld, Oomycetes, Chromalveolata. Synonyms: None. Common names: Twig and leaf blight (EU), Ramorum leaf blight (North America), Sudden Oak Death= SOD (North America), tamme-äkksurm (EE), maladie de l’encre des chênes rouges (FR), mort subite du chêne (FR), tammen äkkikuolema (FI), europæisk visneskimmel (DK, European isolates) / californisk visneskimmel (DK, North American isolates), Plötslig ekdöd (SE), Plötzliches eichensterben (DE), Nagła śmier ć d ębu (POL). Fig 1 . Sporangia of Phytophthora ramorum in soil extract water, photo by Arja Lilja. 1 Fig 2 . Branched dendroid-like hyphae of Phytophthora ramorum on the bottom of an agar plate, photo by Arja Lilja. Fig 3. Clamydospore of Phytophthora ramorum , photo by Arja Lilja. Species identification Phytophthora ramorum is a heterothallic species characterized by abundant production of chlamydospores and elongate, ellipsoid, deciduous sporangia. The mean sporangium length was 43.6 µm ± 5.3 with a range from 20-79 µm, and the mean sporangium width 23.9 µm ± 2.6 with a range from 12-40 µm in measurements done by Werres and Kaminski (2005). -

Expressed Sequence Tag Analysis of the Response of Apple
Physiologia Plantarum 133: 298–317. 2008 Copyright ª Physiologia Plantarum 2008, ISSN 0031-9317 Expressed sequence tag analysis of the response of apple (Malus x domestica ‘Royal Gala’) to low temperature and water deficit Michael Wisniewskia,*, Carole Bassetta,*, John Norellia, Dumitru Macarisina, Timothy Artlipa, Ksenija Gasicb and Schuyler Korbanb aUnited States Department of Agriculture – Agricultural Research Service (USDA-ARS), Appalachian Fruit Research Station, 2217 Wiltshire Road, Kearneysville, WV 25430, USA bDepartment of Natural Resources and Environmental Sciences, University of Illinois, 310 ERML, 1201 W. Gregory Drive, Urbana, IL 61801, USA Correspondence Leaf, bark, xylem and root tissues were used to make nine cDNA libraries from *Corresponding author, non-stressed (control) ‘Royal Gala’ apple trees, and from ‘Royal Gala’ trees e-mail: [email protected]; exposed to either low temperature (5°C for 24 h) or water deficit (45% of [email protected] saturated pot mass for 2 weeks). Over 22 600 clones from the nine libraries # Received 26 September 2007; revised 3 were subjected to 5 single-pass sequencing, clustered and annotated using January 2008 BLASTX. The number of clusters in the libraries ranged from 170 to 1430. Regarding annotation of the sequences, BLASTX analysis indicated that within doi: 10.1111/j.1399-3054.2008.01063.x the libraries 65–72% of the clones had a high similarity to known function genes, 6–15% had no functional assignment and 15–26% were completely novel. The expressed sequence tags were combined into three classes (control, low-temperature and water deficit) and the annotated genes in each class were placed into 1 of 10 different functional categories. -

Identification and Evolutionary Relationships of Partial Gene
Identification and evolutionary relationships of partial gene sequences from dehydrin group in three species of cacti Identificación y relaciones evolutivas de secuencias parciales de genes del grupo dehidrina en tres especies de cactáceas Hernández-Camacho S1, E Pérez-Molphe-Balch1, AG Alpuche-Solís2, JF Morales-Domínguez1 Abstract. Dehydrins or Group 2 Late Embryogenesis Abundant Resumen. Las dehidrinas o proteínas abundantes de la embrio- (LEA) proteins play an important role in the response and adapta- génesis tardía (LEA) del grupo 2 juegan un rol importante en la tion to different types of abiotic stresses such as droughts, high salin- respuesta y adaptación a diferentes tipos de estrés abiótico como des- ity and low temperatures. Using PCR techniques, we identified three hidratación, alta salinidad y bajas temperaturas. Usando técnicas de gene fragments that encoded dehydrin-like proteins in three cacti PCR, se identificaron tres fragmentos de genes que codifican para species Opuntia ficus-indica (OpfiDHN-like), Leuchtenbergia prin- proteínas tipo dehidrina de tres especies de cactus: Opuntia ficus- cipis (LepDHN-like) and Mammillaria bombycina (MabDHN-like). indica (OpfiDHN-like), Leuchtenbergia principis (LepDHN-like) y Bioinformatic sequence analysis showed an identity between 96 and Mammillaria bombycina (MabDHN-like). El análisis bioinformático 97% with the Opuntia streptacantha dehydrin 1 (OpsDHN1) gene, de las secuencias mostró que poseen una identidad entre el 96 y 97% demonstrating that the amplified fragments corresponded to dehy- con la secuencia del gen dehidrina 1 (OpsDHN1) de Opuntia strep- drin-like gene sequences, and that the designed oligonucleotides tacantha, demostrando que los fragmentos amplificados correspon- were effective for similar gene amplification in different cacti genera. -

Complete Chloroplast Genome of Japanese Larch (Larix Kaempferi): Insights Into Intraspecific Variation with an Isolated Northern Limit Population
Article Complete Chloroplast Genome of Japanese Larch (Larix kaempferi): Insights into Intraspecific Variation with an Isolated Northern Limit Population Shufen Chen 1, Wataru Ishizuka 2, Toshihiko Hara 3 and Susumu Goto 1,* 1 Education and Research Center, The University of Tokyo Forests, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan; [email protected] 2 Forestry Research Institute, Hokkaido Research Organization, Koushunai, Bibai, Hokkaido 079-0166, Japan; [email protected] 3 Institute of Low Temperature Science, Hokkaido University, Sapporo-city, Hokkaido 060-0819, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-3-5841-5493 Received: 25 July 2020; Accepted: 11 August 2020; Published: 14 August 2020 Abstract: Research Highlights: The complete chloroplast genome for eight individuals of Japanese larch, including from the isolated population at the northern limit of the range (Manokami larch), revealed that Japanese larch forms a monophyletic group, within which Manokami larch can be phylogenetically placed in Japanese larch. We detected intraspecific variation for possible candidate cpDNA markers in Japanese larch. Background and Objectives: The natural distribution of Japanese larch is limited to the mountainous range in the central part of Honshu Island, Japan, with an isolated northern limit population (Manokami larch). In this study, we determined the phylogenetic position of Manokami larch within Japanese larch, characterized the chloroplast genome of Japanese larch, detected intraspecific variation, and determined candidate cpDNA markers. Materials and Methods: The complete genome sequence was determined for eight individuals, including Manokami larch, in this study. -

IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

Diverse Accumulation of Several Dehydrin-Like Proteins in Cauliflower (Brassica Oleracea Var
Rurek BMC Plant Biology 2010, 10:181 http://www.biomedcentral.com/1471-2229/10/181 RESEARCH ARTICLE Open Access Diverse accumulation of several dehydrin-like proteins in cauliflower (Brassica oleracea var. botrytis), Arabidopsis thaliana and yellow lupin (Lupinus luteus) mitochondria under cold and heat stress Michal Rurek Abstract Background: Dehydrins represent hydrophilic proteins acting mainly during cell dehydration and stress response. Dehydrins are generally thermostable; however, the so-called dehydrin-like (dehydrin-related) proteins show variable thermolability. Both groups immunoreact with antibodies directed against the K-segment of dehydrins. Plant mitochondrial dehydrin-like proteins are poorly characterized. The purpose of this study was to extend previous reports on plant dehydrins by comparing the level of immunoprecipitated dehydrin-like proteins in cauliflower (Brassica oleracea var. botrytis), Arabidopsis thaliana and yellow lupin (Lupinus luteus) mitochondria under cold and heat stress. Results: All the analyzed plant species showed constitutive accumulation of thermostable mitochondrial putative dehydrins ranging from 50 to 70 kDa. The mitochondrial dehydrin-like proteins observed in cauliflower and Arabidopsis ranged from 10 to 100 kDa and in lupin imbibed seeds and hypocotyls - from 20 to 90 kDa. Cold treatment increased mainly the accumulation of 10-100 kDa cauliflower and Arabidopsis dehydrin-like proteins, in the patterns different in cauliflower leaf and inflorescence mitochondria. However, in lupin mitochondria, cold affected mainly 25-50 kDa proteins and seemed to induce the appearance of some novel dehydrin-like proteins. The influence of frost stress on cauliflower leaf mitochondrial dehydrin- like proteins was less significant. The impact of heat stress was less significant in lupin and Arabidopsis than in cauliflower inflorescence mitochondria. -
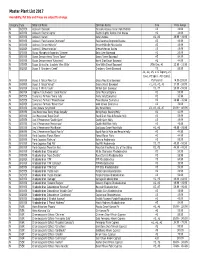
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Cadhn3, a Pepper (Capsicum Annuum L.) Dehydrin Gene Enhances the Tolerance Against Salt and Drought Stresses by Reducing ROS Accumulation
International Journal of Molecular Sciences Article CaDHN3, a Pepper (Capsicum annuum L.) Dehydrin Gene Enhances the Tolerance against Salt and Drought Stresses by Reducing ROS Accumulation Yuan-Cheng Meng 1,†, Hua-Feng Zhang 1,†, Xiao-Xiao Pan 1,†, Nan Chen 1, Hui-Fang Hu 1, Saeed ul Haq 1,2, Abid Khan 1,3 and Ru-Gang Chen 1,4,* 1 College of Horticulture, Northwest A&F University, Yangling 712100, China; [email protected] (Y.-C.M.); [email protected] (H.-F.Z.); [email protected] (X.-X.P.); [email protected] (N.C.); [email protected] (H.-F.H.); [email protected] (S.u.H.); [email protected] (A.K.) 2 Department of Horticulture, The University of Agriculture Peshawar, Peshawar 25130, Pakistan 3 Department of Horticulture, The University of Haripur, Haripur 22620, Pakistan 4 Shaanxi Engineering Research Center for Vegetables, Yangling 712100, China * Correspondence: [email protected]; Tel./Fax: +86-29-8708-2613 † These authors contribute equally to this work. Abstract: Dehydrins (DHNs) play an important role in abiotic stress tolerance in a large number of plants, but very little is known about the function of DHNs in pepper plants. Here, we isolated a Y1SK2-type DHN gene “CaDHN3” from pepper. To authenticate the function of CaDHN3 in salt and Citation: Meng, Y.-C.; Zhang, H.-F.; drought stresses, it was overexpressed in Arabidopsis and silenced in pepper through virus-induced Pan, X.-X.; Chen, N.; Hu, H.-F.; Haq, gene silencing (VIGS). Sub-cellular localization showed that CaDHN3 was located in the nucleus S.u.; Khan, A.; Chen, R.-G. -

Plant Dehydrins —
CELLULAR & MOLECULAR BIOLOGY LETTERS Volume 11 (2006) pp 536 - 556 http://www.cmbl.org.pl DOI: 10.2478/s11658-006-0044-0 Received: 20 March 2006 Revised form accepted: 28 June 2006 © 2006 by the University of Wrocław, Poland PLANT DEHYDRINS – TISSUE LOCATION, STRUCTURE AND FUNCTION TADEUSZ RORAT* Institute of Plant Genetics, PAS, Strzeszyńska 34, 60-479 Poznań, Poland Abstract: Dehydrins (DHNs) are part of a large group of highly hydrophilic proteins known as LEA (Late Embryogenesis Abundant). They were originally identified as group II of the LEA proteins. The distinctive feature of all DHNs is a conserved, lysine-rich 15-amino acid domain, EKKGIMDKIKEKLPG, named the K-segment. It is usually present near the C-terminus. Other typical dehydrin features are: a track of Ser residues (the S-segment); a consensus motif, T/VDEYGNP (the Y-segment), located near the N-terminus; and less conserved regions, usually rich in polar amino acids (the Φ-segments). They do not display a well-defined secondary structure. The number and order of the Y-, S- and K-segments define different DHN sub-classes: YnSKn, YnKn, SKn, Kn and KnS. Dehydrins are distributed in a wide range of organisms including the higher plants, algae, yeast and cyanobacteria. They accumulate late in embryogenesis, and in nearly all the vegetative tissues during normal growth conditions and in response to stress leading to cellular dehydration (e.g. drought, low temperature and salinity). DHNs are localized in different cell compartments, such as the cytosol, nucleus, mitochondria, vacuole, and the vicinity of the plasma membrane; however, they are primarily localized to the cytoplasm and nucleus. -

Phytophthora Ramorum
Forest Health Pest Information Note 3 of 2021 Phytophthora ramorum Updated: 01/07/2021 Background Phytophthora ramorum is a harmful pathogen which is known to have over 180 hosts, many of which are tree spe- cies. Phytophthora ramorum was first noticed in the early 1990s in plant nurseries in Europe and in forests in Cali- fornia. In mainland Europe, it causes a serious blight of ornamental plants, especially Rhododendron, Camellia and Viburnum. In North America it causes a forest disease called Sudden Oak Death, killing millions of Quercus trees in deciduous forests of California and Oregon. It also costs millions annually to the ornamental nursery industry in Europe and North America. In Ireland, Phytophthora ramorum was first detected in 2002 on imported Rhododendron and Viburnum, and in the wild in 2003 on Rhododendron ponticum. In 2010, Phytophthora ramorum was found to be infecting trees in Ire- land, in particular Japanese larch (Larix kaempferi), and has since been recognised as a serious threat as it can cause damage and death of Japanese larch. Sanitation felling of infected Japanese larch stands has been carried- out – based on policy and legislative requirements, in an effort to limit the spread of the disease. By the end of 2020 Phytophthora ramorum had been found in Japanese larch at 56 forest locations. Phytophthora ramorum has also been a major problem for the United Kingdom over the last decade. There have also been limited outbreaks in north western France in Japanese larch in recent years. However, in the rest of the EU, Phytophthora ramorum has not yet proved to be a forest health issue of concern being far more associated with the horticultural nursery trade. -

The New Phytophthora Ramorum Dynamic in Europe: Spread to Larch1
Proceedings of the Sudden Oak Death Fifth Science Symposium The New Phytophthora ramorum Dynamic in Europe: Spread to Larch1 Anna Harris2 and Joan Webber2 Abstract Phytophthora ramorum has been reported from most European Union member states, mainly affecting ornamental plants in nurseries. The most epidemiologically important hosts are those that support abundant sporulation, and, until recently, in Europe this applied primarily to rhododendron and vaccinium. However, following the first findings of P. ramorum on Japanese larch (Larix kaempferi (Lam.) Carrière) in southwest Britain in 2009, it soon became clear that infected foliage of Japanese larch could produce abundant numbers of sporangia, as demonstrated in the laboratory (see Webber, J.F.; Mullett, M.; Brasier, C.M. 2010. Dieback and mortality of plantation Japanese larch (Larix kaempferi) associated with infection by Phytophthora ramorum. New Disease Reports. 22: 19.) and on naturally infected needles, leading to bark infections on larch and other nearby susceptible tree species. To compare the spore-producing potential of larch foliage with other known sporulating hosts (Umbellularia californica (Hook. & Arn.) Nutt. and Rhododendron ponticum L.), laboratory tests were carried out using shoots of Japanese larch, hybrid larch (Larix x eurolepis Henry), and European larch (L. decidua Mill.) challenged with zoospores suspensions of P. ramorum (EU1 lineage). These tests were carried out at different times of year and have shown that sporulation potential varies with larch species, pathogen genotype, and also with the age of the foliage. Japanese larch generally supports the highest levels of sporulation, even exceeding that on U. californica. Sporulation on larch needles can also occur in the absence of any symptoms, particularly early in the season.