Invasive Alien Species Fact Sheet Phytophthora Ramorum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research on the Epidemiology, Ecology and Management of Phytophthora Ramorum in California Forests1
Research on the Epidemiology, Ecology and Management of Phytophthora ramorum in California Forests1 David M. Rizzo2 Key words: disease management, landscape ecology, invasive species Introduction The ultimate goal of Phytophthora ramorum research is to develop disease management strategies. To date, studies have been focused at three management levels: the individual tree, the landscape (or forest stand), and the regional to international scale (Garbelotto and others 2003, Rizzo and Garbelotto 2003, Rizzo and others 2005). I will focus my brief remarks on the forest landscape, possibly the most difficult level to implement disease control strategies (for a more comprehensive discussion see Rizzo and others 2005). From a research perspective, there are four non-exclusive areas that we must continue to focus on in order to facilitate P. ramorum management in forest stands: Put the impacts of sudden oak death in context with expected successional patterns and ecosystem processes within coastal forests For any P. ramorum management proposal to be successful in coastal California forests it must also be put into context with other management goals (e.g. timber extraction, wildlife) and threats (e.g., high fuel loads due to fire suppression, other invasive species) (Rizzo and others 2005). This requires continued study on the ecology of the invaded forests in addition to examining the biology of P. ramorum. This pathogen has invaded three broad forest types in California: mixed-evergreen forests dominated by coast live oak (Quercus agrifolia), mixed-evergreen forests dominated by tanoak (Lithocarpus densiflorus) and Douglas-fir (Pseudotsuga menziesii), and coast redwood (Sequoia sempervirens) forests. With the possible exception of the coast redwood forest type, the ecology of many coastal mixed-evergreen forests has not been well characterized (Barbour and Minnich 2000). -

Susceptibility of Larch, Hemlock, Sitka Spruce, and Douglas-Fir to Phytophthora Ramorum1
Proceedings of the Sudden Oak Death Fifth Science Symposium Susceptibility of Larch, Hemlock, Sitka Spruce, and 1 Douglas-fir to Phytophthora ramorum Gary Chastagner,2 Kathy Riley,2 and Marianne Elliott2 Introduction The recent determination that Phytophthora ramorum is causing bleeding stem cankers on Japanese larch (Larix kaempferi (Lam.) Carrière) in the United Kingdom (Forestry Commission 2012, Webber et al. 2010), and that inoculum from this host appears to have resulted in disease and canker development on other conifers, including western hemlock (Tsuga heterophylla (Raf.) Sarg.), Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), grand fir (Abies grandis (Douglas ex D. Don) Lindl.), and Sitka spruce (Picea sitchensis (Bong.) Carrière), potentially has profound implications for the timber industry and forests in the United States Pacific Northwest (PNW). A clearer understanding of the susceptibility of these conifers to P. ramorum is needed to assess the risk of this occurring in the PNW. Methods An experiment was conducted to examine the susceptibility of new growth on European (L. decidua Mill.), Japanese, eastern (L. laricina (Du Roi) K. Koch), and western larch (L. occidentalis Nutt.); western and eastern hemlock (T. canadensis (L.) Carrière); Sitka spruce; and a coastal seed source of Douglas-fir to three genotypes (NA1, NA2, and EU1) of P. ramorum in 2011. In 2012, a similar experiment was conducted using only the four larch species. Container-grown seedlings or saplings were used in all experiments. Five trees or branches of each species were inoculated with a single isolate of the three genotypes by spraying the foliage with a suspension of zoospores (105/ml). -

Phytophthora Ramorum
USDA’s Cooperative State Research, Education, and Extension Service Web site at www.csrees.usda. gov/Extension/index.html, or consult the blue pages of the telephone book under “U.S. Department of Agriculture, Cooperative State Research, Education, United States Department of Agriculture Animal and Plant Health Inspection Service and Extension Service.” A directory of State plant regulatory officials is available on the National Plant Board Web site at www.nationalplantboard.org/ member/index.shtml. Phytophthora For more information and resources concerning ramorum: Figure 6—Employees from the Maryland Department P. ramorum, visit APHIS’ Web site at of Agriculture’s Plant Protection and Weed Management www.aphis.usda.gov/plant_health/plant_pest_info/ Stopping Division (left) and APHIS’ Plant Protection and Quarantine pram/index.shtml ■ program collect leaf samples from rhododendrons during a national survey for P. ramorum in nurseries. (APHIS photo by the Spread R. Anson Eaglin) Program Aid No. 1842 APHIS has enacted strict regulations in 15 coun- ties along the California and Oregon coastline be- The U.S. Department of Agriculture (USDA) prohibits cause P. ramorum causes significant disease in those discrimination in all its programs and activities on the basis forest environments. For nurseries, preventing the of race, color, national origin, age, disability, and where introduction and establishment of the diseases applicable, sex, marital status, familial status, parental P. ramorum causes is the best defense. status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) How You Can Help Persons with disabilities who require alternative means for communication of program information (Braille, large print, If you suspect that shrubs or other plants are infect- audiotape, etc.) should contact USDA’s TARGET Center at ed with P. -

Complete Chloroplast Genome of Japanese Larch (Larix Kaempferi): Insights Into Intraspecific Variation with an Isolated Northern Limit Population
Article Complete Chloroplast Genome of Japanese Larch (Larix kaempferi): Insights into Intraspecific Variation with an Isolated Northern Limit Population Shufen Chen 1, Wataru Ishizuka 2, Toshihiko Hara 3 and Susumu Goto 1,* 1 Education and Research Center, The University of Tokyo Forests, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan; [email protected] 2 Forestry Research Institute, Hokkaido Research Organization, Koushunai, Bibai, Hokkaido 079-0166, Japan; [email protected] 3 Institute of Low Temperature Science, Hokkaido University, Sapporo-city, Hokkaido 060-0819, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-3-5841-5493 Received: 25 July 2020; Accepted: 11 August 2020; Published: 14 August 2020 Abstract: Research Highlights: The complete chloroplast genome for eight individuals of Japanese larch, including from the isolated population at the northern limit of the range (Manokami larch), revealed that Japanese larch forms a monophyletic group, within which Manokami larch can be phylogenetically placed in Japanese larch. We detected intraspecific variation for possible candidate cpDNA markers in Japanese larch. Background and Objectives: The natural distribution of Japanese larch is limited to the mountainous range in the central part of Honshu Island, Japan, with an isolated northern limit population (Manokami larch). In this study, we determined the phylogenetic position of Manokami larch within Japanese larch, characterized the chloroplast genome of Japanese larch, detected intraspecific variation, and determined candidate cpDNA markers. Materials and Methods: The complete genome sequence was determined for eight individuals, including Manokami larch, in this study. -

IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -
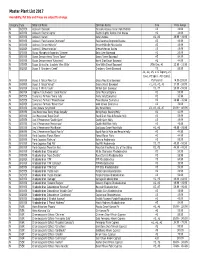
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Phytophthora Ramorum and P. Kernoviae Diseases on Bilberry
Phytophthora ramorum and Phytophthora kernoviae diseases on bilberry (Vaccinium myrtillus) A threat to our woodlands, heathlands and historic gardens Very early symptom showing a brown lesion developing around the leaf node What is it and where is it found? Phytophthora is a devastating fungus-like organism that causes damage to a wide range of trees and plants. Recently two species of Phytophthora – Phytophthora ramorum and Phytophthora kernoviae – have been causing damage to our environment. Why the concern? PLANT DISEASE FACTSHEET Initial findings of the disease were predominantly confined to ornamental plants in nurseries. Since then, findings in the wider environment have continued to spread across the UK after initially being concentrated in the South West. With a large and varied host range, if left unchecked they may change our landscape with the loss of many trees, shrubs and heathland plants. Where is it found? P. ramorum was first identified in 1995 causing the death of oak and tanoak trees in the coastal counties of California in the USA. It was first detected in the UK in 2002 in the nursery trade and has since spread to the wider environment including historic gardens, parks, woodlands and heathlands. The first finding of P. ramorum on the heathland plant Vaccinium myrtillus (bilberry/blaeberry) in the wild was confirmed in January 2009. More recently, in August 2009, the pathogen was identified on Japanese larch trees at sites in South West England. P. kernoviae was first discovered in 2003 causing severe damage to rhododendron and beech trees in Cornwall. The disease has been found in woodlands, gardens and a small number of nurseries with outbreaks mainly confined to the South West. -

Phytophthora Ramorum: a Guide for Oregon Nurseries Hazel A
OREGON STATE UNIVERSITY EXTENSION SERVICE Phytophthora Ramorum: A Guide for Oregon Nurseries Hazel A. Daniels, Jennifer Parke, Jay W. Pscheidt and Chris Benemann Summary ¾ Phytophthora ramorum became a federally quarantined pathogen in 2005. ¾ This pathogen causes the diseases known as ramorum leaf and twig blight, and sudden oak death. ¾ In the United States, the regulated host list includes more than 150 plant species in 37 genera. ¾ Repeated shipments of P. ramorum-infested plants by nurseries have resulted in spread of the disease. In 2019, for example, nationwide trace investigations were triggered due to the detection of P. ramorum positive plants in commerce. The infected plants originated from two nurseries in Washington state and Canada and were shipped to 18 states. Photo: Jennifer Parke, © Oregon State University ¾ Nurseries should be vigilant in monitoring plant Figure 1: Ramorum shoot dieback and leaf blight on Viburnum x material brought in from external sources. bodnantense ‘Dawn’. ¾ Management efforts in Pacific Northwest nurseries (Quercus spp.) in the San Francisco Bay Area were dying are focused on eradicating the pathogen when it is from a new disease, sudden oak death. The cause of both found and preventing new infections. sudden oak death and the nursery diseases was found to be Phytophthora ramorum, which was formally described ¾ If P. ramorum is found in your nursery, and you ship in 2001. material outside of Oregon, you will be required to sign a cooperative agreement with the United By 2001, P. ramorum had spread to 10 counties in States Department of Agriculture and Oregon coastal California. The pathogen was also detected Department of Agriculture. -

Emergence of the Sudden Oak Death Pathogen Phytophthora Ramorum
Review Emergence of the sudden oak death pathogen Phytophthora ramorum 1 2 3 4 Niklaus J. Gru¨ nwald , Matteo Garbelotto , Erica M. Goss , Kurt Heungens and 5 Simone Prospero 1 Horticultural Crops Research Laboratory, United States Department of Agriculture (USDA) Agricultural Research Service, Corvallis, OR 97330, USA 2 Department of Environmental Science, Policy and Management, University of California, Berkeley, CA 94720, USA 3 Emerging Pathogens Institute and Department of Plant Pathology, University of Florida, Gainesville, FL 32611, USA 4 Institute for Agricultural and Fisheries Research (ILVO), Plant Sciences Unit – Crop Protection, 9820 Merelbeke, Belgium 5 Swiss Federal Institute for Forest, Snow and Landscape Research WSL, 8903 Birmensdorf, Switzerland The recently emerged plant pathogen Phytophthora can only be formed when the two mating types encounter ramorum is responsible for causing the sudden oak and mate. P. ramorum is heterothallic and produces death epidemic. This review documents the emergence sporangia and chlamydospores. Oospores have not been of P. ramorum based on evolutionary and population observed in the known geographic distribution of genetic analyses. Currently infection by P. ramorum P. ramorum. occurs only in Europe and North America and three Phytophthora ramorum is adapted to cool temperatures clonal lineages are distinguished: EU1, NA1 and NA2. with optimal growth at 20 8C [3]. The life cycle of P. Ancient divergence of these lineages supports a scenario ramorum resembles that of other splash-dispersed Phy- in which P. ramorum originated from reproductively tophthora species [1]. Deciduous sporangia are formed on isolated populations and underwent at least four global the surface of infected leaves or twigs and, depending upon migration events. -

Impacts of Phytophthora Ramorum Canker and Other Agents in Sonoma County Forests1
Impacts of Phytophthora ramorum Canker and Other Agents in Sonoma County 1 Forests Tedmund J. Swiecki2 and Elizabeth A. Bernhardt2 Abstract To study impacts of sudden oak death (SOD), a lethal bark canker disease caused by Phytophthora ramorum, we established permanent plots in Sonoma County forest types at risk of SOD. Baseline stand and tree health data were collected in 2001 and the plots were reassessed in 2004. The 250 plots (0.02 ha each) were located at 11 study locations in stands containing Quercus agrifolia, Q. kelloggii, or Lithocarpus densiflorus as the dominant hardwood species. By 2004, P. ramorum canker symptoms developed at two locations that lacked symptoms in 2001, leading to new tree mortality at one of these locations. Between 2001 and 2004, plot level incidence of P. ramorum canker increased from 29 to 40 percent of plots containing L. densiflorus and from 2 to 10 percent in plots containing Q. kelloggii. Plots with Q. agrifolia showed a slight drop in P. ramorum canker (from 9 to 7 percent of plots) due to apparent symptom remission in trees at one location. Between 2001 and 2004, the percentage of trees with P. ramorum canker symptoms increased at three of four locations with symptomatic SOD canker hosts. Mortality due to both P. ramorum and other agents increased at 9 of 11 study locations between 2001 and 2004. Among SOD canker hosts that died during this period, mortality was due to P. ramorum in 4 of 16 Q. kelloggii, 7 of 18 Q. agrifolia, and 18 of 50 L. densiflorus. In most study locations, annual background mortality unrelated to P. -

Understandingphytophthora Ramorum in Irish Larch Forests
Understanding Phytophthora ramorum in Irish larch forests Authors: Richard O’ Hanlon, Helen Grogan, Teagasc, Dublin, Ireland Alistair McCracken, Lourdes de la Mata Saez, Agri-Food and Biosciences Institute (AFBI), Belfast, Northern Ireland Fiona Doohan, Jianguang Jia, University College, Dublin (UCD), Ireland Tom Harrington, University of Limerick, Ireland This project was funded by the Department of Agriculture, Food and Josephine Brennan, James Choiseul, Department of Agriculture, the Marine’s competitive research programmes. Food and the Marine (DAFM), Kildare, Ireland Phytophthora ramorum is a fungus-like pathogen that poses In Ireland, P. ramorum was first detected in 2002 on imported a significant threat to forests in the island of Ireland. Of Rhododendron and Viburnum at several garden centres and in 2003 most concern is the recent host range expansion from low it was detected in the wild, infecting Rhododendron ponticum. In value Rhododendron to Japanese larch in commercial forest 2010 it was observed on Japanese larch in Ireland and Northern plantations in Ireland and Northern Ireland. This factsheet Ireland, and has since been recognised as a serious threat to forestry. P. ramorum describes new information on the biology and detection of Under current plant health policy, control has resulted in the pathogen and provides updated information on disease the removal of more than 1300 ha of larch forests on the island of Ireland and 17,000 ha in Britain. Phytophthora ramorum has been management. a regulated quarantine pathogen within the EU since 2002, with confirmed infected trees in forests removed and susceptible hosts KEY RECOMMENDATIONS within a 250 meter radius also removed as a precautionary measure. -

Phytophthora Ramorum
Forest Health Pest Information Note 3 of 2021 Phytophthora ramorum Updated: 01/07/2021 Background Phytophthora ramorum is a harmful pathogen which is known to have over 180 hosts, many of which are tree spe- cies. Phytophthora ramorum was first noticed in the early 1990s in plant nurseries in Europe and in forests in Cali- fornia. In mainland Europe, it causes a serious blight of ornamental plants, especially Rhododendron, Camellia and Viburnum. In North America it causes a forest disease called Sudden Oak Death, killing millions of Quercus trees in deciduous forests of California and Oregon. It also costs millions annually to the ornamental nursery industry in Europe and North America. In Ireland, Phytophthora ramorum was first detected in 2002 on imported Rhododendron and Viburnum, and in the wild in 2003 on Rhododendron ponticum. In 2010, Phytophthora ramorum was found to be infecting trees in Ire- land, in particular Japanese larch (Larix kaempferi), and has since been recognised as a serious threat as it can cause damage and death of Japanese larch. Sanitation felling of infected Japanese larch stands has been carried- out – based on policy and legislative requirements, in an effort to limit the spread of the disease. By the end of 2020 Phytophthora ramorum had been found in Japanese larch at 56 forest locations. Phytophthora ramorum has also been a major problem for the United Kingdom over the last decade. There have also been limited outbreaks in north western France in Japanese larch in recent years. However, in the rest of the EU, Phytophthora ramorum has not yet proved to be a forest health issue of concern being far more associated with the horticultural nursery trade.