Macrotis Lagotis, in Queensland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sociogenetic Organization of the Red Honey Ant (Melophorus Bagoti)
insects Article Sociogenetic Organization of the Red Honey Ant (Melophorus bagoti) Nathan Lecocq de Pletincx * and Serge Aron Evolutionary Biology & Ecology, Université Libre de Bruxelles, B-1050 Brussels, Belgium; [email protected] * Correspondence: [email protected]; Tel.: +32-472015404 Received: 6 October 2020; Accepted: 2 November 2020; Published: 4 November 2020 Simple Summary: Monogamy is thought to be a major factor having favored the evolution of a non- reproductive worker caste in eusocial insects because it optimizes the relatedness among colony members. However, polyandry evolved secondarily in a large number of species. By increasing the genetic diversity within colonies, multiple mating can enhance worker task efficiency and resistance to diseases. Polyandry may also favor social harmony by reducing worker–queen conflict over male parentage. This is because in colonies headed by a single, multiple-mated queen, workers can increase their inclusive fitness by rearing their brothers (queen sons) rather than their nephews (offspring of other workers). Using DNA microsatellites, we showed that nests of the red honey ant, Melophorus bagoti, are headed by a single, multiple-mated queen. Morphometric analyses revealed two distinct worker subcastes: majors and minors; yet, we found no relationship between worker patriline and worker subcaste. Workers can produce males in the presence of the queen under natural conditions, which contrasts with predictions of inclusive fitness theory. Abstract: Kin selection and inclusive fitness are thought to be key factors explaining the reproductive altruism displayed by workers in eusocial insect species. However, when a colony’s queen has mated with <2 males, workers may increase their fitness by producing their own male offspring. -

Report to Office of Water Science, Department of Science, Information Technology and Innovation, Brisbane
Lake Eyre Basin Springs Assessment Project Hydrogeology, cultural history and biological values of springs in the Barcaldine, Springvale and Flinders River supergroups, Galilee Basin and Tertiary springs of western Queensland 2016 Department of Science, Information Technology and Innovation Prepared by R.J. Fensham, J.L. Silcock, B. Laffineur, H.J. MacDermott Queensland Herbarium Science Delivery Division Department of Science, Information Technology and Innovation PO Box 5078 Brisbane QLD 4001 © The Commonwealth of Australia 2016 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 3.0 Australia (CC BY) licence Under this licence you are free, without having to seek permission from DSITI or the Commonwealth, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the source of the publication. For more information on this licence visit http://creativecommons.org/licenses/by/3.0/au/deed.en Disclaimer This document has been prepared with all due diligence and care, based on the best available information at the time of publication. The department holds no responsibility for any errors or omissions within this document. Any decisions made by other parties based on this document are solely the responsibility of those parties. Information contained in this document is from a number of sources and, as such, does not necessarily represent government or departmental policy. If you need to access this document in a language other than English, please call the Translating and Interpreting Service (TIS National) on 131 450 and ask them to telephone Library Services on +61 7 3170 5725 Citation Fensham, R.J., Silcock, J.L., Laffineur, B., MacDermott, H.J. -

The Nature of Northern Australia
THE NATURE OF NORTHERN AUSTRALIA Natural values, ecological processes and future prospects 1 (Inside cover) Lotus Flowers, Blue Lagoon, Lakefield National Park, Cape York Peninsula. Photo by Kerry Trapnell 2 Northern Quoll. Photo by Lochman Transparencies 3 Sammy Walker, elder of Tirralintji, Kimberley. Photo by Sarah Legge 2 3 4 Recreational fisherman with 4 barramundi, Gulf Country. Photo by Larissa Cordner 5 Tourists in Zebidee Springs, Kimberley. Photo by Barry Traill 5 6 Dr Tommy George, Laura, 6 7 Cape York Peninsula. Photo by Kerry Trapnell 7 Cattle mustering, Mornington Station, Kimberley. Photo by Alex Dudley ii THE NATURE OF NORTHERN AUSTRALIA Natural values, ecological processes and future prospects AUTHORS John Woinarski, Brendan Mackey, Henry Nix & Barry Traill PROJECT COORDINATED BY Larelle McMillan & Barry Traill iii Published by ANU E Press Design by Oblong + Sons Pty Ltd The Australian National University 07 3254 2586 Canberra ACT 0200, Australia www.oblong.net.au Email: [email protected] Web: http://epress.anu.edu.au Printed by Printpoint using an environmentally Online version available at: http://epress. friendly waterless printing process, anu.edu.au/nature_na_citation.html eliminating greenhouse gas emissions and saving precious water supplies. National Library of Australia Cataloguing-in-Publication entry This book has been printed on ecoStar 300gsm and 9Lives 80 Silk 115gsm The nature of Northern Australia: paper using soy-based inks. it’s natural values, ecological processes and future prospects. EcoStar is an environmentally responsible 100% recycled paper made from 100% ISBN 9781921313301 (pbk.) post-consumer waste that is FSC (Forest ISBN 9781921313318 (online) Stewardship Council) CoC (Chain of Custody) certified and bleached chlorine free (PCF). -

Level 1 Fauna Survey of the Gruyere Gold Project Borefields (Harewood 2016)
GOLD ROAD RESOURCES LIMITED GRUYERE PROJECT EPA REFERRAL SUPPORTING DOCUMENT APPENDIX 5: LEVEL 1 FAUNA SURVEY OF THE GRUYERE GOLD PROJECT BOREFIELDS (HAREWOOD 2016) Gruyere EPA Ref Support Doc Final Rev 1.docx Fauna Assessment (Level 1) Gruyere Borefield Project Gold Road Resources Limited January 2016 Version 3 On behalf of: Gold Road Resources Limited C/- Botanica Consulting PO Box 2027 BOULDER WA 6432 T: 08 9093 0024 F: 08 9093 1381 Prepared by: Greg Harewood Zoologist PO Box 755 BUNBURY WA 6231 M: 0402 141 197 T/F: (08) 9725 0982 E: [email protected] GRUYERE BOREFIELD PROJECT –– GOLD ROAD RESOURCES LTD – FAUNA ASSESSMENT (L1) – JAN 2016 – V3 TABLE OF CONTENTS SUMMARY 1. INTRODUCTION .....................................................................................................1 2. SCOPE OF WORKS ...............................................................................................1 3. RELEVANT LEGISTALATION ................................................................................2 4. METHODS...............................................................................................................3 4.1 POTENTIAL VETEBRATE FAUNA INVENTORY - DESKTOP SURVEY ............. 3 4.1.1 Database Searches.......................................................................................3 4.1.2 Previous Fauna Surveys in the Area ............................................................3 4.1.3 Existing Publications .....................................................................................5 4.1.4 Fauna -

Heritage of the Birdsville and Strzelecki Tracks
Department for Environment and Heritage Heritage of the Birdsville and Strzelecki Tracks Part of the Far North & Far West Region (Region 13) Historical Research Pty Ltd Adelaide in association with Austral Archaeology Pty Ltd Lyn Leader-Elliott Iris Iwanicki December 2002 Frontispiece Woolshed, Cordillo Downs Station (SHP:009) The Birdsville & Strzelecki Tracks Heritage Survey was financed by the South Australian Government (through the State Heritage Fund) and the Commonwealth of Australia (through the Australian Heritage Commission). It was carried out by heritage consultants Historical Research Pty Ltd, in association with Austral Archaeology Pty Ltd, Lyn Leader-Elliott and Iris Iwanicki between April 2001 and December 2002. The views expressed in this publication are not necessarily those of the South Australian Government or the Commonwealth of Australia and they do not accept responsibility for any advice or information in relation to this material. All recommendations are the opinions of the heritage consultants Historical Research Pty Ltd (or their subconsultants) and may not necessarily be acted upon by the State Heritage Authority or the Australian Heritage Commission. Information presented in this document may be copied for non-commercial purposes including for personal or educational uses. Reproduction for purposes other than those given above requires written permission from the South Australian Government or the Commonwealth of Australia. Requests and enquiries should be addressed to either the Manager, Heritage Branch, Department for Environment and Heritage, GPO Box 1047, Adelaide, SA, 5001, or email [email protected], or the Manager, Copyright Services, Info Access, GPO Box 1920, Canberra, ACT, 2601, or email [email protected]. -

The Functions and Evolution of Social Fluid Exchange in Ant Colonies (Hymenoptera: Formicidae) Marie-Pierre Meurville & Adria C
ISSN 1997-3500 Myrmecological News myrmecologicalnews.org Myrmecol. News 31: 1-30 doi: 10.25849/myrmecol.news_031:001 13 January 2021 Review Article Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae) Marie-Pierre Meurville & Adria C. LeBoeuf Abstract Trophallaxis is a complex social fluid exchange emblematic of social insects and of ants in particular. Trophallaxis behaviors are present in approximately half of all ant genera, distributed over 11 subfamilies. Across biological life, intra- and inter-species exchanged fluids tend to occur in only the most fitness-relevant behavioral contexts, typically transmitting endogenously produced molecules adapted to exert influence on the receiver’s physiology or behavior. Despite this, many aspects of trophallaxis remain poorly understood, such as the prevalence of the different forms of trophallaxis, the components transmitted, their roles in colony physiology and how these behaviors have evolved. With this review, we define the forms of trophallaxis observed in ants and bring together current knowledge on the mechanics of trophallaxis, the contents of the fluids transmitted, the contexts in which trophallaxis occurs and the roles these behaviors play in colony life. We identify six contexts where trophallaxis occurs: nourishment, short- and long-term decision making, immune defense, social maintenance, aggression, and inoculation and maintenance of the gut microbiota. Though many ideas have been put forth on the evolution of trophallaxis, our analyses support the idea that stomodeal trophallaxis has become a fixed aspect of colony life primarily in species that drink liquid food and, further, that the adoption of this behavior was key for some lineages in establishing ecological dominance. -

East Kimberley Impact Assessment Project
East Kimberley Impact Assessment Project HISTORICAL NOTES RELEVANT TO IMPACT STORIES OF THE EAST KIMBERLEY Cathie Clement* East Kimberley Working Paper No. 29 ISBN O 86740 357 8 ISSN 0816...,6323 A Joint Project Of The: Centre for Resource and Environmental Studies Australian National University Australian Institute of Aboriginal Studies Anthropology Department University of Western Australia Academy of the Social Sciences in Australia The aims of the project are as follows: 1. To compile a comprehensive profile of the contemporary social environment of the East Kimberley region utilising both existing information sources and limited fieldwork. 2. Develop and utilise appropriate methodological approaches to social impact assessment within a multi-disciplinary framework. 3. Assess the social impact of major public and private developments of the East Kimberley region's resources (physical, mineral and environmental) on resident Aboriginal communities. Attempt to identify problems/issues which, while possibly dormant at present, are likely to have implications that will affect communities at some stage in the future. 4. Establish a framework to allow the dissemination of research results to Aboriginal communities so as to enable them to develop their own strategies for dealing with social impact issues. 5. To identify in consultation with Governments and regional interests issues and problems which may be susceptible to further research. Views expressed in the Projecfs publications are the views of the authors, and are not necessarily shared by the sponsoring organisations. Address correspondence to: The Executive Officer East Kimberley Project CRES, ANU GPO Box4 Canberra City, ACT 2601 HISTORICAL NOTES RELEVANT TO IMPACT STORIES OF THE EAST KIMBERLEY Cathie Clement* East Kimberley Working Paper No. -

100 the SOUTH-WEST CORNER of QUEENSLAND. (By S
100 THE SOUTH-WEST CORNER OF QUEENSLAND. (By S. E. PEARSON). (Read at a meeting of the Historical Society of Queensland, August 27, 1937). On a clear day, looking westward across the channels of the Mulligan River from the gravelly tableland behind Annandale Homestead, in south western Queensland, one may discern a long low line of drift-top sandhills. Round more than half the skyline the rim of earth may be likened to the ocean. There is no break in any part of the horizon; not a landmark, not a tree. Should anyone chance to stand on those gravelly rises when the sun was peeping above the eastem skyline they would witness a scene that would carry the mind at once to the far-flung horizons of the Sahara. In the sunrise that western region is overhung by rose-tinted haze, and in the valleys lie the purple shadows that are peculiar to the waste places of the earth. Those naked, drift- top sanddunes beyond the Mulligan mark the limit of human occupation. Washed crimson by the rising sun they are set Kke gleaming fangs in the desert's jaws. The Explorers. The first white men to penetrate that line of sand- dunes, in south-western Queensland, were Captain Charles Sturt and his party, in September, 1845. They had crossed the stony country that lies between the Cooper and the Diamantina—afterwards known as Sturt's Stony Desert; and afterwards, by the way, occupied in 1880, as fair cattle-grazing country, by the Broad brothers of Sydney (Andrew and James) under the run name of Goyder's Lagoon—and the ex plorers actually crossed the latter watercourse with out knowing it to be a river, for in that vicinity Sturt describes it as "a great earthy plain." For forty miles one meets with black, sundried soil and dismal wilted polygonum bushes in a dry season, and forty miles of hock-deep mud, water, and flowering swamp-plants in a wet one. -

Boulia Shire Council General Meeting
Boulia Shire Council General Meeting Wednesday 23rd August 2017 Photos courtesy of Councillor Rebecka Britton ALL COMMUNICATIONS MUST TELEPHONE: (07)[Type 4746 3188 here] BE ADDRESSED TO FACSIMILE: (07) 4746 3136 THE CHIEF EXECUTIVE OFFICER, BOULIA SHIRE COUNCIL, EMAIL: 18 HERBERT ST, [email protected] BOULIA. QLD. 4829 ABN: 20 492 088 398 BOULIA SHIRE COUNCIL 16th August 2017 NOTICE FOR AN ORDINARY MEETING OF COUNCIL NOTICE is hereby given that an ORDINARY MEETING of the Boulia Shire Council will be held on Wednesday 23rd August 2017 at the Boulia Airport Terminal commencing at 9-00am for the transaction of the following business. Councillor attendance via teleconference is deemed to be in attendance at the meeting with full voting rights. 1. Meeting opening with the acknowledgment of Country and Traditional Owners 2. Present 3. Apologies / Leave of Absence 4. Declaration of Material Personal Interest / Conflict of Interest 5. Register of Interests – new / or changes recorded 6. Confirmation of Minutes from previous meeting Pg.1 7. Action List – Council resolutions and action items Pg.9 8. Reports from advisory committees (nil) 9. Mayoral Minutes 10. Notice of Motion 11. Request to address Council in a public forum 12. Petition – if tabled 13. Pitta Pitta representation (when requested) 14. REPORTS: ENGINEERS REPORT – GBA CONSULTING Item E1 Engineering Services Report – Newsbrief Pg.16 DIRECTOR OF WORKS AND OPERATIONS Item DWO1 Grants Work Status Summary Pg.22 Item DWO2 Director of Works and Operations Report Pg.28 INFORMATION -

The Economics of Road Transport of Beef Cattle
THE ECONOMICS OF ROAD TRANSPORT OF BEEF CATTLE NORTHERN TERRITORY AND QUEENSLAND CHANNEL COUNTRY BUREAU OF AGRICULTURAL ECONOMICS \CANBERRA! AUSTRALIA C71 A.R.A.PURA SEA S5 CORAL SEA NORTHERN TER,RITOR 441I AND 'go \ COUNTRY DARWIN CHANNEL Area. ! Arnhem Land k OF 124,000 S9.mla Aborig R e ), QUEENSLAND DARWIN 1......../L5 GULF OF (\11 SHOWING CARPENTARIA NUMBERS —Zr 1, AREAS AND CATTLE I A N ---- ) TAKEN AT 30-6-59 IN N:C.AND d.1 31-3-59 IN aLD GULF' &Lam rol VI LO Numbers 193,000 \ )14 LEGEND Ar DISTRIT 91, 200 Sy. mls. ••• The/ Elarkl .'-lc • 'Tx/at:viand ER Area , 94 000'S‘frn Counb-v •• •1 411111' == = == Channal Cattle Numbers •BARKLY• = Fatizning Araas 344,0W •• 4* • # DISTRICT • Tannin! r Desert TABLELANDS, 9.4• • 41" amoowea,1 •• • • Area, :NV44. 211,800Sq./nil -N 4 ••• •Cloncurry ALICE NXil% SOCITIf PACIFIC DISTRI W )• 9uches `N\ OCEAN Cattle \ •Dajarra, -r Number 28,000 •Winbon 4%,,\\ SPRINGS A rn/'27:7 0 liazdonnell Ji Ranges *Alice Springs Longreach Simpson DISTRICT LCaWe Desert Numbers v 27.1000 ITh Musgra Ranges. T ullpq -_,OUNTRY JEJe NuTber4L. S A 42Anc SAE al- (gCDET:DWaD [2 ©MU OniVITELE2 NORTHERN TERRITORY AND QUEENSLAND CHANNEL COUNTRY 1959 BUREAU OF AGRICULTURAL ECONOMICS CANBERRA AUSTRALIA 4. REGISTERED AT THE G.P.O. SYDNEY FOR TRANSMISSION BY POST AS A BOOK PREFACE. The Bureau of Agricultural Economics has undertaken an investigation of the economics of road transport of beef cattle in the remote parts of Australia inadequately served by railways. The survey commenced in 1958 when investigations were carried out in the pastoral areas of Western Australia and a report entitled "The Economics of Road Transport of Beef Cattle - Western Australian Pastoral Areas" was subsequently issued. -
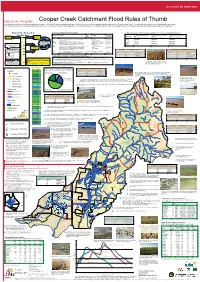
Cooper Creek Catchment Flood Rules of Thumb This Guide Has Used the Best Information Available at Present
QueenslandQueenslandthethe Smart Smart State State How to use this guide: Cooper Creek Catchment Flood Rules of Thumb This guide has used the best information available at present. It is intended to help you assess what type of flood is likely to occur in your area and indicate what amount of feed you might expect. You may wish to record your own flooding guides on the map. You can add more value to this guide by participating in an MLA EDGEnetwork Grazing Land Management (GLM) training package. GLM training helps you identify land types and flood zones and to develop a grazing management plan for your property Amount of rain needed Channel Country Flood descriptions Estimated Summer Flood Pasture Growth in the Channel Country Floodplains. Frequently flooded plains Occasionally flooded plains Swamps and depressions for flooding Flood type Description Land Hydrology Pasture growth Isolated Systems which supports: Flood type (C1) (C2) (C3) Widespread Widespread Rain 100 mm Localised Rain “HANDY” to flooded “GOOD” flood Then increases (kg DM/ha of useful feed) (kg DM/ha of useful feed) (kg DM/ha of useful feed) 95 “GOOD” flood Good Good floods are similar to handy floods, but cover a much higher C1, C3, C2 Flooded across most of 85 - 100% of to proportion of the floodplain (75% or more) and grow more feed per floodplains potential cattle Good 1200-2500 1500-3500 4500-8000 90 area than a handy flood. 80-100% inundation numbers 85 IF in 24-72 hrs Handy Handy floods occur when the water escapes from the gutters, C1, C3 Pushing out of gutters across 45 - 85% of Handy 750-1500 100-250 3500-6500 80 PRIOR, rains of (or useful) connecting up to form the large sheets of water. -
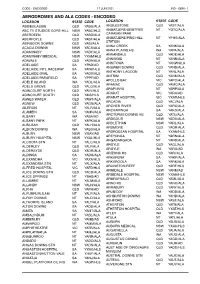
Aerodromes and Ala Codes
CODE - ENCODED 17 JUN 2021 IND - GEN - 1 AERODROMES AND ALA CODES - ENCODED LOCATION STATE CODE LOCATION STATE CODE ABBIEGLASSIE QLD YABG/ALA ANGLESTONE QLD YAST/ALA ABC TV STUDIOS GORE HILL NSW YABC/HLS ANMATJERE/GEMTREE NT YGTC/ALA ABERDEEN QLD YABD/ALA CARAVAN PARK ABERFOYLE QLD YABF/ALA ANMATJERE/PINE HILL NT YPHS/ALA STATION ABINGDON DOWNS QLD YABI/ALA ANNA CREEK SA YANK/ALA ACACIA DOWNS NSW YACS/ALA ANNA PLAINS HS WA YAPA/ALA ADAMINABY NSW YADY/ALA ANNANDALE QLD YADE/ALA ADAMINABY MEDICAL NSW YXAM/HLS ANNINGIE NT YANN/ALA ADAVALE QLD YADA/ALA ANNITOWA NT YANW/ALA ADELAIDE SA YPAD/AD ANSWER DOWNS QLD YAND/ALA ADELAIDE INTL RACEWAY SA YAIW/HLS ANTHONY LAGOON NT YANL/ALA ADELAIDE OVAL SA YAOV/HLS ANTRIM QLD YANM/ALA ADELAIDE/PARAFIELD SA YPPF/AD APOLLO BAY VIC YAPO/ALA ADELE ISLAND WA YADL/ALA ARAMAC QLD YAMC/ALA ADELS GROVE QLD YALG/ALA ARAPUNYA NT YARP/ALA AGINCOURT NORTH QLD YAIN/HLS ARARAT VIC YARA/AD AGINCOURT SOUTH QLD YAIS/HLS ARARAT HOSPITAL VIC YXAR/HLS AGNES WATER QLD YAWT/ALA ARCADIA QLD YACI/ALA AGNEW QLD YAGN/ALA ARCHER RIVER QLD YARC/ALA AILERON NT YALR/ALA ARCKARINGA SA YAKG/ALA ALAMEIN SA YAMN/ALA ARCTURUS DOWNS HS QLD YATU/ALA ALBANY WA YABA/AD ARDGOUR NSW YADU/ALA ALBANY PARK NT YAPK/ALA ARDLETHAN NSW YARL/ALA ALBILBAH QLD YALH/ALA ARDMORE QLD YAOR/ALA ALBION DOWNS WA YABS/ALA ARDROSSAN HOSPITAL SA YXAN/HLS ALBURY NSW YMAY/AD AREYONGA NT YARN/ALA ALBURY HOSPITAL NSW YXAL/HLS ARGADARGADA NT YARD/ALA ALCOOTA STN NT YALC/ALA ARGYLE QLD YAGL/ALA ALDERLEY QLD YALY/ALA ARGYLE WA YARG/AD ALDERSYDE QLD YADR/ALA ARIZONA HS