Water Column Monitoring 2017 Environmental Monitoring Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
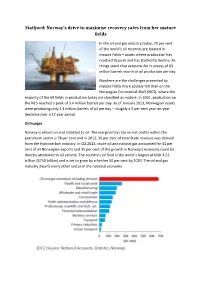
Statfjord: Norway's Drive to Maximise Recovery Rates from Her Mature Fields
Statfjord: Norway’s drive to maximise recovery rates from her mature fields In the oil and gas industry today, 70 per cent of the world's oil reserves are located in mature fields – assets where production has reached its peak and has started to decline. As things stand that accounts for in excess of 63 million barrels-worth of oil production per day. Nowhere are the challenges presented by mature fields more acutely felt than on the Norwegian Continental Shelf (NCS), where the majority of the 69 fields in production today are classified as mature. In 2001, production on the NCS reached a peak of 3.4 million barrels per day. As of January 2013, Norwegian assets were producing only 1.4 million barrels of oil per day – roughly a 5 per cent year-on-year decrease over a 12 year period. Oil hunger Norway is reliant on and addicted to oil. The marginal tax rate on net profits within the petroleum sector is 78 per cent and in 2012, 30 per cent of total State revenue was derived from the hydrocarbon industry. In Q2 2013, crude oil and natural gas accounted for 41 per cent of all Norwegian exports and 35 per cent of the growth in Norway’s economy could be directly attributed to oil activity. The country’s oil fund is the world’s largest at NOK 4.52 trillion ($750 billion) and is set to grow by a further 50 per cent by 2020. The oil and gas industry dwarfs every other sector in the national economy. -

Offshore Petroleum Exploitation and Environmental Protection: the International and Norwegian Response
Comments OFFSHORE PETROLEUM EXPLOITATION AND ENVIRONMENTAL PROTECTION: THE INTERNATIONAL AND NORWEGIAN RESPONSE Degradation of the marine environment inevitably accompa- nies the pursuit of offshore hydrocarbons. This Comment first ex- amines the international legal regime which establishes both jurisdictionalrights of exploitation and the coastal States' obli- gation to mitigate concomitant environmental harms. The writer then focuses on a single coastal State, Norway, to study a domes- tic response to the conflicting needs of offshore exploitation and environmental protection. Issues attending offshore petroleum exploitation confront the world community as it progresses toward a new international law of the sea.' Alternative energy sources must be developed,2 yet 1. By the end of the century, offshore oil could account for nearly half of the annual world production of some seventy billion barrels. R. HALumAN, TowARDs AN ENVIRoNmENTALLY SouND LAw OF THE SEA 3 (1974). Therefore, according to one author, the value of offshore petroleum and gas production will exceed that of the world's fish catch to become the most important marine resource. That being so, one of the problems which arises is to determine how the different interests which States, and the international community as a whole, have in the marine environment, are to be accommodated in a legal order. Hardy, Offshore Development and Marine Pollution, 1 OCEAN DEv. & INT'L L 239, 240 (1973). See generally, NATIONAL PETROLEUM CouuNc, LAW OF THE SEA (1973). See Finlay & McKnight, Law of the Sea:t Its Impact on the InternationalEnergy Crisis,6 INT'L L. & POL'Y Bus. 639 (1974). 2. "It is estimated that by 1990 the free world will use as much as 100 million barrels of oil .. -

WCM 2003 Summary. Rev. 2005.Pdf
RF-Akvamiljø Børseth, Jan Fredrik1 and Tollefsen, Knut-Erik2 1) RF-Akvamiljø, 2) NIVA Water Column Monitoring 2003 - Summary Report Report RF – 2004/039 Project number: 715 1686 Project Quality Assurance: Odd-Ketil Andersen, RF-Akvamiljø Ketil Hylland, NIVA Client(s): Norsk Hydro Produksjon AS on behalf of OLF WCM coordination group Distribution restriction: Confidential © This document may only be reproduced with the permission of RF or the client. RF - Rogaland Research has a certified Quality System in compliance with the standard NS - EN ISO 9001 RF – Rogaland Research. http://www.rf.no Preface This is the summary report of the Water Column Monitoring 2003 project. The project has been a collaboration project between RF-Akvamiljø and Norwegian Institute for Water Research (NIVA) with several sub-contractors. The sub-contractors have been The Centre for Environment, Fisheries & Aquaculture Science (CEFAS), University of the Basque Country (UPV/EHU), University of Stockholm. Other contributors to the project, but coordinated directly from Norsk Hydro and OLF have been Ocean Climate a/s and the Norwegian Institute of Marine Research, represented by the crew onboard R/V Sarsen. In addition to the expertise given by Bjørn Serigstad, Ocean Climate a/s with regard to the technical part of the fish and mussel caging, he contributed with valuable comments to the cruise report. This has been the first regular water column monitoring survey in the North Sea and it was based on guidelines given from the BECPELAG Workshop in 2001. We would like to thank a number of people at the different companies and institutes that have contributed to make this a valuable project. -

10Fields in Production
eng_fakta_2005_kap10 12-04-05 15:26 Side 66 10 Fields in production eng_fakta_2005_kap10 12-04-05 15:26 Side 67 Keys to tables in chapters 10–12 Interests in fields do not necessarily correspond with interests in the individual production licences (unitised fields or ones for which the sliding scale has been exercised have a different composition of interests than the production licence). Because interests are shown up to two decimal places, licensee holdings in a field may add up to less than 100 percent. Interests are shown at 1 January 2005. “Recoverable reserves originally present” refers to reserves in resource categories 0, 1, 2 and 3 in the NPD’s classification system (see the definitions below). “Recoverable reserves remaining” refers to reserves in resource categories 1, 2 and 3 in the NPD’s classification system (see the definitions below). Resource category 0: Petroleum sold and delivered Resource category 1: Reserves in production Resource category 2: Reserves with an approved plan for development and operation Resource category 3: Reserves which the licensees have decided to develop FACTS 2005 67 eng_fakta_2005_kap10 12-04-05 15:26 Side 68 Southern North Sea The southern part of the North Sea sector became important for the country at an early stage, with Ekofisk as the first Norwegian offshore field to come on stream, more than 30 years ago. Ekofisk serves as a hub for petroleum operations in this area, with surrounding developments utilising the infrastructure which ties it to continental Europe and Britain. Norwegian oil and gas is exported from Ekofisk to Teesside in the UK and Emden in Germany respectively. -

Reassessment of the Norway North Sea Demersal Fisheries
PUBLIC CERTIFICATION REPORT FOR THE Reassessment of the Norway North Sea demersal fisheries Norges Fiskarlag Report No.: 2017-024, Rev. 4 Date: 2018.06.11 Certificate code: F-DNV-60011 Report type: Public Certification Report for the DNV GL – Business Assurance Report title: Reassessment of the Norway North Sea demersal fisheries DNV GL Business Assurance Customer: Norges Fiskarlag, Pirsenteret, 7462 Trondheim Norway AS Contact person: Tor Bjørklund Larsen Veritasveien 1 Date of issue: 2018.06.11 1322 HØVIK, Norway Project No.: PRJC-504565-2014-MSC-NOR Tel: +47 67 57 99 00 Organisation unit: ZNONO418 http://www.dnvgl.com Report No.: 2017-024, Rev.4 Certificate No.: F-DNV-60011 Objective: Re-assessment of the Norway North Sea demersal fisheries against MSC Fisheries Standards v2.0 (Assessment tree v.1.3). Prepared by: Verified by: Mrs. Sandhya Chaudhury Sigrun Bekkevold Team Leader and Chain of Custody expert Hans Lassen Team expert P1 Lucia.Revenga Giertych Team expert P2 Geir Hønneland Team expert P3 Copyright © DNV GL 2014. All rights reserved. This publication or parts thereof may not be copied, reproduced or transmitted in any form, or by any means, whether digitally or otherwise without the prior written consent of DNV GL. DNV GL and the Horizon Graphic are trademarks of DNV GL AS. The content of this publication shall be kept confidential by the customer, unless otherwise agreed in writing. Reference to part of this publication which may lead to misinterpretation is prohibited. DNV GL Distribution: Keywords: ☒ Unrestricted distribution (internal and external) MSC Fisheries, Norway, North sea, saithe, Pollachius ☐ Unrestricted distribution within DNV GL virens, cod, Gadus morhua, haddock, ☐ Limited distribution within DNV GL after 3 years Melanogrammus aeglefinus, hake (European), ☐ No distribution (confidential) Merluccius merluccius, re-assessment ☐ Secret Rev. -

Environmental Statement for the Gjøa to FLAGS Pipeline
Environmental Statement for the Gjøa to FLAGS pipeline PL 153 Gjøa Vega Gas export Power from shore Oil export November 2006 PL 153 Gjøa Environmental Statement for the Gjøa to FLAGS pipeline November 2006 Standard Information Sheet Project name: Gjøa to FLAGS Gas Export Pipeline DTI Project reference: D/3325/2006 Type of project: Field Development Undertaker Name: Statoil ASA Address: Statoil ASA N-4035 Stavanger Norway Licensees/Owners: RWE Dea Norge AS 8% A/S Norske Shell 12% Statoil ASA 20% Petoro AS 30% Gaz de France Norge AS 30% Short description: Statoil are proposing to install a new 28” (ID) gas export pipeline between the Gjøa SEMI and FLAGS, as part of the Gjøa project. The new export pipeline will be connected to a new Gjøa semi-submersible platform via two 12” risers. The pipeline will be connected to FLAGS at the Tampen Link connection, via a new Hot Tap Tee-piece. All connections at Gjøa and at FLAGS will be stabilised using gravel and rock and will be fitted with protective structures. Dates Anticipated commencement May 2007 of works: Date and reference number Not applicable of any earlier Statement related to this project: Significant environmental Physical Presence of Vessels impacts identified: Anchoring of vessels during pipeline Installation Pipeline installation Physical presence of the pipeline and subsea structures Emissions from anodes Accidental spills of diesel Statement Prepared By: Statoil ASA BMT Cordah Limited, Aberdeen i PL 153 Gjøa Environmental Statement for the Gjøa to FLAGS pipeline November 2006 This -

Not Enough for Christmas?
(Periodicals postage paid in Seattle, WA) TIME-DATED MATERIAL — DO NOT DELAY Travel Arts & Style Meet a new A kayak journey Det er en uimotståelig erfaring at den norske vinter bør tilbringes Norwegian-American to remember ved Middelhavet. author –Jens Bjørneboe Read more on page 9 Read more on page 12 Norwegian American Weekly Vol. 123 No. 45 December 7, 2012 Established May 17, 1889 • Formerly Western Viking and Nordisk Tidende $1.50 per copy News in brief Find more at blog.norway.com Not enough for Christmas? News A new book about HM King Tariffs cut on Harald and his career as a sailor shows that the sport has been a imported place for the king to relax, unwind pork to ensure and be himself. It’s on the water where the king has been able Norway will have to just be Harald. “He doesn’t want to be the king there,” says enough ribbe for author Jon Amtrup to VG. The Christmas book talks about a situation at sea where there was no hierarchy, but a place where everybody was VG equal and helped each other. (Norway Post) Anticipating a shortage of International Relations ribbe from Norwegian pigs, the Ukraine’s Prime Minister was on Norwegian Agricultural Author- an official visit to Norway for ity (Statens landruksforvaltning – the first time. The Norwegian- SLF) lowered tariffs on imported Ukrainian business forum pork to ensure that Norwegians marked the historic visit. Sectors will have enough of the traditional like energy, fish and seafood, dinner item for Christmas celebra- shipbuilding, IT and tourism were tions. -

Norway: Selected Issues; IMF Country Report 15/250; July 30, 2015
IMF Country Report No. 15/250 NORWAY SELECTED ISSUES September 2015 This Selected Issues paper on Norway was prepared by a staff team of the International Monetary Fund as background documentation for the periodic consultation with the member country. It is based on the information available at the time it was completed on July 30, 2015. Copies of this report are available to the public from International Monetary Fund Publication Services PO Box 92780 Washington, D.C. 20090 Telephone: (202) 623-7430 Fax: (202) 623-7201 E-mail: [email protected] Web: http://www.imf.org Price: $18.00 per printed copy International Monetary Fund Washington, D.C. © 2015 International Monetary Fund NORWAY SELECTED ISSUES July 30, 2015 Approved By Prepared By Tom Dorsey, Giang Ho, and Kazuko Shirono. Philip Gerson N CONTENTS NORWAY’S ECONOMY WITH A MATURING OIL AND GAS INDUSTRY _______________ 3 A. The Direct Contribution of Oil and Gas to the Norwegian Economy ____________________ 3 B. The Fiscal Contribution of Oil and Gas to the Mainland Economy: The Fiscal Rule and the Sovereign Wealth Fund ________________________________________________________________ 4 C. Real Sector Links Between the Offshore and Mainland Economies _____________________ 7 D. Policies for Norway’s Future __________________________________________________________ 12 BOXES 1. Measuring Oil and Gas Sector Demand in Mainland Economy __________________________ 8 2. Investment and Production in Norwegian Fields ______________________________________ 10 3. What Determines Oil Investment and Output? ________________________________________ 11 FIGURE 1. Oil and Gas Related Incomes, 2014 ______________________________________________________ 9 REFERENCES ____________________________________________________________________________ 14 THE HOUSING BOOM AND HOUSEHOLD DEBT IN NORWAY _______________________ 15 A. House Prices and Household Debt ____________________________________________________ 15 B. -

Oil and Gas Workers' Views on Industry Conditions and the Energy Transition
OFFSHORE Oil and gas workers’ views on industry conditions and the energy transition Platform is an environmental and social justice collective based in London with campaigns focused on the global oil industry, fossil fuel finance and building capacity toward climate justice and energy democracy. Platform’s Just Transition campaign seeks a well-managed, worker-led phase-out of oil and gas production in the North Sea. The campaign is focused on increasing worker consultation and power over policy decisions related to an oil and gas phase-out. Friends of the Earth Scotland (FoES) campaigns for socially just solutions to environmental problems and to create a green economy; for a world where everyone can enjoy a healthy environment and a fair share of the earth’s resources. Climate change is the greatest threat to this aim, which is why FoES is calling for a just transition to a 100% renewable Scotland through a well-managed phase-out of oil and gas production in the UK. Greenpeace stands for positive change through action to defend the natural world and promote peace. Greenpeace investigates, documents and exposes the causes of environmental destruction. A just transition is essential to move to an environmentally sustainable economy without leaving workers in polluting industries behind. This survey and report was conducted and written by Gabrielle Jeliazkov, Platform, Ryan Morrison, FoES, and Mel Evans, Greenpeace. Thank you to all the offshore workers who gave their thoughts and time to this project. ACRONYMS BEIS Department for Business, -

Water Column Monitoring 2004 Summary Report
REPORT SNO 4993-2005 Water Column Monitoring 2004 Summary Report Photo: Hylland, Lang Norwegian Institute for Water Research – an institute in the Environmental Research Alliance of Norway REPORT Main Office Regional Office, Sørlandet Regional Office, Østlandet Regional Office, Vestlandet Akvaplan-NIVA A/S P.O. Box 173, Kjelsås Televeien 3 Sandvikaveien 41 Nordnesboder 5 N-0411 Oslo, Norway N-4879 Grimstad, Norway N-2312 Ottestad, Norway N-5008 Bergen, Norway N-9005 Tromsø, Norway Phone (47) 22 18 51 00 Phone (47) 37 29 50 55 Phone (47) 62 57 64 00 Phone (47) 55 30 22 50 Phone (47) 77 68 52 80 Telefax (47) 22 18 52 00 Telefax (47) 37 04 45 13 Telefax (47) 62 57 66 53 Telefax (47) 55 30 22 51 Telefax (47) 77 68 05 09 Internet: www.niva.no Title Serial No. Date Water Column Monitoring 2004 March, 2005 Summary Report Report No. Sub-No. Pages Price 4993-2005 59 + appendix Author(s) Topic group Distribution Ketil Hylland Ionan Marigomez Anders Ruus Lennart Balk Rolf C. Sundt Alexandra Abrahamsson Geographical area Printed Steve Feist Janina Baršienė North Sea NIVA Client(s) Client ref. Statoil ASA on behalf of the OLF WCM coordination group Abstract This report presents results from the Water Column Monitoring 2004, carried out in collaboration between NIVA and RF-Akvamiljø, with sub-contractors. The objective of the survey was to assess the extent to which discharges from Statfjord B affect organisms living in the water column. The study was designed to monitor bioaccumulation and biomarker responses in organisms held in cages in the vicinity of the platform. -

Ices Cm 2007/I:05
ICES CM 2007/I:05 Not to be cited without prior reference to the authors ICES CM 2007/I:05 Theme Session on Effects of hazardous substances on ecosystem health in coastal and brackish-water ecosystems: present research, monitoring strategies, and future requirements (I) Environmental genotoxicity studies in marine fish and mussels Janina Baršienė, Aleksandras Rybakovas, Laura Andreikėnaitė Institute of Ecology of Vilnius University, Akademijos 2, 08412 Vilnius, Lithuania, [tel: +370 68260979, fax: +370 52729257, e-mail: [email protected]] Abstract: A growing concern over the presence of genotoxins in marine media, there is a rising need to elaborate sensitive methods for the assessment genetic damage in indigenous organisms. It has been developed different methods for the detection of both double- and single-strand breaks of DNA, DNA-adducts, micronuclei formation, chromosome aberrations. The micronucleus (MN) test has been widely used in vivo assay and was proved as simple to perform, sensitive enough and fast test to detect genomic alterations due to clastogenic effects and impairments of mitotic spindle caused by aneuploidogenic poisons. Main objective of the present study is to identify regularities of genotoxicity in marine indigenous organisms in situ, under experimental caging, deployment and laboratory conditions. Peculiarities of MN formation were investigated in various cells of fish and mussel species inhabiting geographically and ecologically different zones of the Baltic and North Seas. Active monitoring approach (fish and mussel caging) applied to assess MN induction in certain polluted areas of the North Sea. MN test validation was performed in multiple controlled exposures at IRIS (Norway) marine experimental center. The wide-range MN investigations indicated specific responses in relation to species, tissue, environmental temperature, contaminant type and concentration, duration of exposure, distance from contamination source. -

FACTS 2006 7 Foreword by the Director General of the Norwegian Petroleum Directorate, Gunnar Berge
FACTS THE NORWEGIAN PETROLEUM SECTOR 2006 Ministry of Petroleum and Energy Visiting address: Einar Gerhardsens plass 1 Postal address: P O Box 8148 Dep, NO-0033 Oslo Tel +47 22 24 90 90 Fax +47 22 24 95 65 www.mpe.dep.no (English) www.oed.dep.no (Norwegian) E-mail: [email protected] Norwegian Petroleum Directorate Visiting address: Prof. Olav Hanssens vei 10 Postal address: P O Box 600, NO-4003 Stavanger Tel +47 51 87 60 00 Fax +47 51 55 15 71/+47 51 87 19 35 www.npd.no E-mail: [email protected] Editors: Ane Dokka (Ministry of Petroleum and Energy) and Øyvind Midttun (Norwegian Petroleum Directorate) Edition completed: March 2006 Layout/design: Janne N’Jai (NPD)/PDC Tangen Cover illustration: Janne N’Jai (NPD) Paper: Cover: Munken Lynx 240g, inside pages: Uni Matt 115g Printer: PDC Tangen Circulation: 8,000 New Norwegian/7,000 English New Norwegian translator Åshild Nordstrand, English translator TranslatørXpress AS by Rebecca Segebarth ISSN 1502-5446 Foreword by the Minister of Petroleum and Energy, Odd Roger Enoksen Status of petroleum sector and its value for Record-high activity in 2005 the Norwegian society The level of activity on the Norwegian continental shelf The petroleum sector is extremely important to was very high in 2005. More than 250 million stand- Norway. This industry is responsible for one ard cubic metres of oil equivalents were produced, fourth of all value creation in the country, and equal to the annual energy consumption of more than more than one fourth of the state’s revenues.