A 50-Year-Old Man with Desmoid-Type Fibromatosis of Upper Extremity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomical Variants in the Termination of the Cephalic Vein Stoyan Novakov1*, Elena Krasteva2
Institute of Experimental Morphology, Pathology and Anthropology with Museum Bulgarian Anatomical Society Acta morphologica et anthropologica, 25 (3-4) Sofia • 2018 Anatomical Variants in the Termination of the Cephalic Vein Stoyan Novakov1*, Elena Krasteva2 1 Department of Anatomy, Histology and Embryology, 2Department of Propaedeutics of Surgical Di- seases, Medical Faculty, Medical University of Plovdiv * Corresponding author e-mail: [email protected] Jugulocephalic vein is atavistic structure which is very rare. The low incidence of the variations of the cephalic vein in deltopectoral triangle and its position on the anterior surface of the clavicle and the neck doesn’t make it less important for the clinical practice. Phylo- and ontogenesis explain the formation of the above mentioned variations. We followed the pattern of the cephalic vein in its proximal part and termination to describe possible variations. In this long term study on 140 upper limbs of 70 cadavers, 4 or 2,9% of the cephalic veins were variable. The direct empting of the cephalic vein into internal jugular is an exception with few descriptions at the moment. The rareness of this anatomical variation doesn’t make it less important for clinical practice. It is described as a possible obstacle in catheter implantation, clavicle fractures and creation of arteriovenous fistula in patients on hemodialysis. Key words:cadavers, human anatomy variation, cephalic vein, external jugular vein, jugulocephalic vein Introduction Cephalic vein (CV) belongs to the group of superficial veins of the upper limb. It usually forms over the anatomical snuff-box on the radial side of the wrist from the radial end of the dorsal venous plexus. -

Infraclavicular Topography of the Brachial Plexus Fascicles in Different Upper Limb Positions
Int. J. Morphol., 34(3):1063-1068, 2016. Infraclavicular Topography of the Brachial Plexus Fascicles in Different Upper Limb Positions Topografía Infraclavicular de los Fascículos del Plexo Braquial en Diferentes Posiciones del Miembro Superior Daniel Alves dos Santos*; Amilton Iatecola*; Cesar Adriano Dias Vecina*; Eduardo Jose Caldeira**; Ricardo Noboro Isayama**; Erivelto Luis Chacon**; Marianna Carla Alves**; Evanisi Teresa Palomari***; Maria Jose Salete Viotto**** & Marcelo Rodrigues da Cunha*,** ALVES DOS SANTOS, D.; IATECOLA, A.; DIAS VECINA, C. A.; CALDEIRA, E. J.; NOBORO ISAYAMA, R.; CHACON, E. L.; ALVES, M. C.; PALOMARI, E. T.; SALETE VIOTTO, M. J. & RODRIGUES DA CUNHA, M. Infraclavicular topography of the brachial plexus fascicles in different upper limb positions. Int. J. Morphol., 34 (3):1063-1068, 2016. SUMMARY: Brachial plexus neuropathies are common complaints among patients seen at orthopedic clinics. The causes range from traumatic to occupational factors and symptoms include paresthesia, paresis, and functional disability of the upper limb. Treatment can be surgical or conservative, but detailed knowledge of the brachial plexus is required in both cases to avoid iatrogenic injuries and to facilitate anesthetic block, preventing possible vascular punctures. Therefore, the objective of this study was to evaluate the topography of the infraclavicular brachial plexus fascicles in different upper limb positions adopted during some clinical procedures. A formalin- preserved, adult, male cadaver was used. The infraclavicular and axillary regions were dissected and the distance of the brachial plexus fascicles from adjacent bone structures was measured. No anatomical variation in the formation of the brachial plexus was observed. The metric relationships between the brachial plexus and adjacent bone prominences differed depending on the degree of shoulder abduction. -

Hypofractionated Irradiation of Infra
Guenzi et al. Radiation Oncology (2015) 10:177 DOI 10.1186/s13014-015-0480-y RESEARCH Open Access Hypofractionated irradiation of infra-supraclavicular lymph nodes after axillary dissection in patients with breast cancer post-conservative surgery: impact on late toxicity Marina Guenzi1, Gladys Blandino1*, Maria Giuseppina Vidili3, Deborah Aloi1, Elena Configliacco1, Elisa Verzanini1, Elena Tornari1, Francesca Cavagnetto2 and Renzo Corvò1 Abstract Background: The aim of the present work was to analyse the impact of mild hypofractionated radiotherapy (RT) of infra-supraclavicular lymph nodes after axillary dissection on late toxicity. Methods: From 2007 to 2012, 100 females affected by breast cancer (pT1- T4, pN1-3, pMx) were treated with conservative surgery, Axillary Node Dissection (AND) and loco-regional radiotherapy (whole breast plus infra-supraclavicular fossa). Axillary lymph nodes metastases were confirmed in all women. The median age at diagnosis was 60 years (range 34–83). Tumors were classified according to molecular characteristics: luminal-A 59pts(59%),luminal-B24pts(24%),basal-like10pts(10%),Her-2like7pts(7%).82pts(82%)received hormonal therapy, 9 pts (9 %) neo-adjuvant chemotherapy, 81pts (81 %) adjuvant chemotherapy. All patients received a mild hypofractionated RT: 46 Gy in 20 fractions 4 times a week to whole breast and infra- supraclavicular fossa plus an additional weekly dose of 1,2 Gy to the lumpectomy area. The disease control and treatment related toxicity were analysed in follow-up visits. The extent of lymphedema was analysed by experts in Oncological Rehabilitation. Results: Within a median follow-up of 50 months (range 19–82), 6 (6 %) pts died, 1 pt (1 %) had local progression disease, 2 pts (2 %) developed distant metastasis and 1 subject (1 %) presented both. -

Originalarticle the Patterns of the Cephalic Veins Termination
OriginalArticle The Patterns of the Cephalic Veins Termination Vasana Plakornkul, DVM., M.Sc., Chayanit Manoonpol, M.Sc. Department of Anatomy, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand. ABSTRACT Objective: To study and classify the types of the terminations of the cephalic veins in the Thais and compare the percent count of each type between male and female, right and left sides. Methods: The ending part of the cephalic veins were dissected and classified in Thai cadavers. Each type was shown by photograph, diagram, number and percent count. Results: The cephalic vein, studied from 208 upper extremities, had three types of termination. Type I, cephalic vein terminated in axillary vein; type II termination was external jugular vein; type III termination was axillary vein and external jugular vein. Conclusion: The termination of cephalic veins were shown and classified into three types and sex had no influence on the patterns of cephalic vein termination. Keywords: Cephalic vein; termination Siriraj Med J 2006; 58: 1204-1207 E-journal: http://www.sirirajmedj.com ephalic vein is a large superficial vein of the triangle and the lower part of occipital triangle, the termi- upper extremities. It originates over the anatomi- nations of the cephalic vein were classified and shown in cal snuffbox at the base of the thumb and drains photographs (Fig 3,4) and diagrams (Fig 1,2). Besides, the Cblood from the dorsal venous plexus.1 At forearm, the number and percent count of each type also were shown cephalic vein ascends in the anterolateral part to the in Table 1,2 and Fig 5. -

Innervation of the Clavicular Part of the Deltoid Muscle by the Lateral Pectoral Nerve
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Innervation of the clavicular part of the deltoid muscle by the lateral pectoral nerve Larionov, Alexey ; Yotovski, Peter ; Link, Karl ; Filgueira, Luis Abstract: INTRODUCTION: The innervation pattern of the clavicular head of the deltoid muscle and its corresponding topography were investigated via cadaveric dissection in the present study, focusing on the lateral pectoral nerve. MATERIALS AND METHODS: Fifty-eight upper extremities were dissected and the nerve supplies to the deltoid muscle and the variability of the lateral pectoral and axillary nerves, including their topographical patterns, were noted. RESULTS: The clavicular portion of the deltoid muscle received a deltoid branch from the lateral pectoral nerve in 86.2% of cases. Two topographical patterns of the lateral pectoral nerve were observed, depending on the branching level from the brachial plexus: a proximal variant, where the nerve entered the pectoral region undern the clavicle, and a distal variant, where the nerve entered the pectoral region from the axillary fossa around the caudal border of the pectoralis minor. These dissection findings were supported by histological confirmation of peripheral nerve tissue entering the clavicular part of the deltoid muscle. CONCLUSION: The topographical variations of the lateral pectoral nerve are relevant for orthopedic and trauma surgeons and neurologists. These new data could revise the interpretation of deltoid muscle atrophy and of thoracic outlet and pectoralis minor compression syndromes. They could also explain the residual anteversion function of the arm after axillary nerve injury and deficiency, which is often thought to be related to biceps brachii muscle function. -

Physical Examination from a Nutritional Standpoint Faith D Ottery, MD, Phd FACN
1 For more information please visit www.pt-global.org Physical Examination from a Nutritional Standpoint Faith D Ottery, MD, PhD FACN History and physical examination are considered the cornerstones of patient diagnosis. Unfortunately, the nutritional aspects of the physical examination -- beyond the global aspects of obesity or cachexia -- are frequently overlooked or under-appreciated. The primary basis for this is a lack of clinician education in this area. Global aspects of physical examination The global aspects of physical examination include one’s first impression of the patient on global examination. This goes beyond the acronym commonly found in the medical record “WDWNWF” for “well- developed, well-nourished white female”. It does include an assessment of general body composition (in terms of muscle, fat, and fluid status) and signs of altered nutritional need (e.g., pressure ulcers or open wounds) or status (e.g., nutrient deficiency). The physical examination includes both measurable and subjective/observable aspects. The most important measurable parameters include height and weight. For accurate assessment over time, it is imperative that these are, in fact, actually measured rather than simply accepting the height or weight reported by the patient. As people age, they tend to lose height but rarely report this. Additionally, the obese or weight-gaining patient tends to underestimate their body weight, while the weight losing patient will often overestimate his/her current weight. If a patient has consistently had involuntary weight loss, the rapidity of weight change as well as the pattern of weight change are particularly important in terms of prognosis. The observable aspects general body composition include an assessment of general muscle and fat mass status as well as evaluation of fluid status. -

179.Full.Pdf
THE AMERICAN JOURNAL OF CANCER A Continuation of The Journal of Cancer Research VOLUMEXXXVI JUNE, 1939 NUMBER2 -_________ CARCINOMA CUTIS ERIK POPPE From the General Department of the Norwegian Radium Hospital, Oslo (Chief: Rolf Bull Engelstnd, M.D.) and thc Hospital's Laboratory for Pathology (Chief: Professor Leiv Kreyberg, M.D.) Histologically three types of skin carcinoma are recognized : the squamous- cell, the basal-cell, and the intermediate. A brief review of these well known forms will suffice. ( 1) The squamous-cell carcinoma is distinguished histologically by dif- ferentiation of the tumor cells, sometimes to a considerable degree, with well marked intercellular bridges and horny pearl formation (Fig. 1). The more malignant forms are less highly differentiated, with nuclei of various sizes, hyperchromasia, and many mitoses. (2) The basal-cell carcinoma is reminiscent of the basal-cell layer in the epidermis or, even more, of the epithelium of young hair sheaths. The nucleus of the tumor cell is spindle-shaped and the cytoplasm is sparse. The appearance of the basal-cell carcinoma varies somewhat. The form most frequently seen consists of solid cords of atypical epithelium outlined by a highly differentiated columnar-cell layer with deeply staining spindle- shaped nuclei (Fig. 2). The tumor cells of the deeper layers are less dif- ferentiated and their nuclei are paler. Some tumors show an adamantinoid type of growth, the cell strands being lined by similar columnar epithelium (Fig. 3). In others there is an alveolar arrangement of the tumor paren- chyma (Fig. 4) suggesting glandular structures. In some of this group a substance resembling mucus is present in the loose connective tissue between the epithelial cords. -

1Chest and Neck
Cambridge University Press 978-0-521-72809-6 - Atlas of Musculoskeletal Ultrasound Anatomy, Second Edition Mike Bradley and Paul O’Donnell Excerpt More information Chapter 1 Chest and neck Supraclavicular fossa This is an ill-defined area at the inferior aspect of the posterior triangle of the neck. It is bounded by the clavicle inferiorly, sternomastoid muscle medially and trapezius postero- laterally. The floor is muscular, comprising levator scapulae, splenius and the three scalene muscles. Contents Accessory nerve Omohyoid External jugular vein Lymph nodes Subclavian artery Brachial plexus 1 © in this web service Cambridge University Press www.cambridge.org Cambridge University Press 978-0-521-72809-6 - Atlas of Musculoskeletal Ultrasound Anatomy, Second Edition Mike Bradley and Paul O’Donnell Excerpt More information Chapter 1: Chest and neck Scalene muscles Scalenus anterior Origin: anterior tubercles cervical vertebrae 3–6. Insertion: scalene tubercle first rib. Scalenus medius Origin: posterior tubercles cervical vertebrae 2–7. Insertion: first rib, posterior to subclavian groove. Scalenus posterior Origin: as part of scalenus medius. Insertion: second rib. Sternomastoid Omohyoid Internal Right lobe jugular vein thyroid Trachea Scalenus anterior Posterior Anterior Phrenic nerve Carotid artery Fig. 1.1. Surface and radiographic anatomy of the sternomastoid. TS, anterior supraclavicular fossa, probe over sternomastoid. 2 © in this web service Cambridge University Press www.cambridge.org Cambridge University Press 978-0-521-72809-6 - Atlas of Musculoskeletal Ultrasound Anatomy, Second Edition Mike Bradley and Paul O’Donnell Excerpt More information Supraclavicular fossa Cords of brachial plexus Sternomastoid Posterior Anterior Scalenus medius and posterior Scalenus anterior Transverse process Fig. 1.2. -

Infraclavicular Brachial Plexus Block in Adults: a Comprehensive Review Based on a Unifed Nomenclature System
Journal of Anesthesia (2019) 33:463–477 https://doi.org/10.1007/s00540-019-02638-0 REVIEW ARTICLE Infraclavicular brachial plexus block in adults: a comprehensive review based on a unifed nomenclature system An‑Chih Hsu1,6 · Yu‑Ting Tai1,2,6 · Ko‑Huan Lin3 · Han‑Yun Yao1 · Han‑Liang Chiang4,5 · Bing‑Ying Ho1 · Sheng‑Feng Yang1 · Jui‑An Lin1,2,6 · Ching‑Lung Ko1 Received: 27 January 2018 / Accepted: 28 March 2019 / Published online: 10 May 2019 © Japanese Society of Anesthesiologists 2019 Abstract Over the last decade, considerable progress has been made regarding infraclavicular brachial plexus block (ICB) in adults, especially since the introduction of ultrasound guidance. The advancements in ICB have been attributed to the development of various approaches to improve the success rate and reduce complications. This has also necessitated a unifed nomencla- ture system to facilitate comparison among diferent approaches. This review aimed to propose an anatomical nomenclature system by classifying ICB approaches into proximal and distal ones to aid future research and provide practice advisories according to recent updates. We also comprehensively discuss various aspects of this nomenclature system. Our review suggests that ultrasound-guided ICB should be categorized as an advanced technique that should be performed under super- vision and dual guidance. For one-shot block, the conventional distal approach is still preferred but should be modifed to follow ergonomic practice, with the arm in the proper position. For continuous ICB, the proximal approach is promising for reducing local anesthetic volume and increasing efcacy. Nevertheless, further studies are warranted in this direction. We provide practice advisories to maximize safety and minimize adverse events, and recommend designing future studies on ICB according to these fndings based on the unifed nomenclature system. -

Topographical Anatomy of the Upper Limb. Axillary Fossa
Topographical anatomy of the upper limb. Axillary fossa Dr. Szuák András 2019.02.12. Today • We don’t learn the upper limb over and over! • Synthesize our knowledge • New - regions Regions of upper limb • Infraclavicular fossa (region) [1,2] • Axillary region/fossa [3] • Scapular region [6] • Deltoid region [5] • Anterior region of arm [7] • Posterior region of arm [8] • Anterior region of elbow [10] • Posterior region of elbow [11] • Anterior region of forearm [12] • Posterior region of forearm [13] • Anterior region of wrist [17] • Posterior region of wrist [18] • Palm [14] • Dorsum of hand [15] • Radial fossa [16] edge of deltoid m. Borders clavicle line through acromion and 1st thoracic vertebra edge of deltoid m. line from inf. angle of scapula 3 cm above olecranon 3 cm below olecranon horizontal line through ulnar head horizontal line through basis of metacarpals horizontal line through carpal eminences Axillary fossa • Anterior: pectoralis major, pectoralis minor • Posterior: latissimus dorsi, subscapularis, teres major (minor) med./lat. axillary hiatus • Medial: serratus anterior • Lateral: surgical neck of humerus, coracobrachials, short head of biceps brachii Axillary artery • superior thoracic a. • thoraco-acromial a. • acromial br. • clavicular br. • deltoid br. • pectoral br. • lateral thoracic a. • subscapular a. • thoracodorsal a. • circumflex scapular a. • anterior circumflex humeral a. • posterior circumflex humeral a. Axillary artery • superior thoracic a. • thoraco-acromial a. • acromial br. • clavicular br. • deltoid br. • pectoral br. • lateral thoracic a. • subscapular a. • thoracodorsal a. • circumflex scapular a. • anterior circumflex humeral a. • posterior circumflex humeral a. Brachial plexus Lymph nodes Anterior region of arm Posterior region of arm Anterior region of elbow Posterior region of elbow Anterior region of forearm Posterior region of forearm Anterior region of wrist Posterior region of wrist Palm Dorsum of hand Radial fossa Thank you for your attention!. -
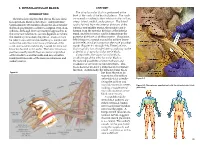
INFRACLAVICULAR BLOCK ANATOMY the Infraclavicular Block Is Performed at the INTRODUCTION Level of the Cords of the Brachial Plexus
9. INFRACLAVICULAR BLOCK ANATOMY The infraclavicular block is performed at the INTRODUCTION level of the cords of the brachial plexus. The cords The infraclavicular brachial plexus block is ideal are named according to their relation to the axillary for operations distal to the elbow. Adequate time artery: lateral, medial, and posterior. The lateral (approximately 20 minutes) should be allowed after cord is formed from the anterior divisions of the the block placement to achieve a surgical level of an- superior and middle trunks, the medial cord is esthesia. Although there are multiple approaches to formed from the anterior division of the inferior the infraclavicular block, success depends on where trunk, and the posterior cord is formed from the the needle tip stimulates the plexus. Caution must posterior divisions of all three trunks. The plexus, be taken to ensure that the needle tip is maintained which begins to spread around the axillary artery within the infraclavicular fossa at the level of the at this level, is not as compact as the more proximal cords and not directed distally toward the terminal trunks (Figures 9-1 through 9-3). Therefore, this branches located in the axilla. The latter erroneous block typically has a longer latency, and may not be position usually results from excessive angulation as dense, as a supraclavicular nerve block. of the needle toward the axilla and may result in Compared to the supraclavicular block, inadequate blockade of the musculocutaneous and an advantage of the infraclavicular block is axillary nerves. the reduced possibility of pneumothorax and avoidance of cervical vascular structures. -
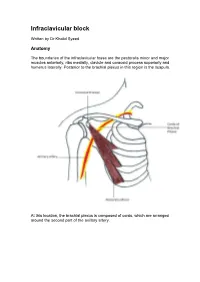
Infraclavicular Block
Infraclavicular block Written by Dr Khalid Syeed Anatomy The boundaries of the infraclavicular fossa are the pectoralis minor and major muscles anteriorly, ribs medially, clavicle and coracoid process superiorly and humerus laterally. Posterior to the brachial plexus in this region is the scapula. At this location, the brachial plexus is composed of cords, which are arranged around the second part of the axillary artery. The sheath surrounding the plexus is delicate and contains the subclavian/axillary artery and vein. The axillary vein is commonly located caudad and medial to the axillary artery. The axillary and musculocutaneous nerves leave the sheath at or before the coracoid process in 50% of patients. The plexus, which begins to spread around the axillary artery at this level, is not as compact as the more proximal trunks. Therefore, this block typically has longer latency, and may not be as dense as a supraclavicular nerve block. The infraclavicular nerve block was first described by Raj et al in 1973. A number of approaches have since been described but only 2 seem to have stood the test of time – the sub coracoid approach and the vertical infraclavicular block. The sub coracoid infraclavicular block is well suited for ultrasound guided local anaesthetic placement. This intermediate technique block approaches the brachial plexus at a level above the departure of the axillary and musculocutaneous nerves, thus providing “whole arm” anaesthesia without the need for additional, more distal, nerve blocks. Indications Surgery of the elbow, forearm, wrist and hand. Territories blocked At the level of the cords-Immediately medial to the coracoid process, the lateral cord of the plexus lies superior and lateral, the posterior cord lies posterior and the medial cord lies posterior and medial to the axillary artery.