Isotopes Are Atoms of the Same Element That Have Different Masses Due to Different Numbers of Neutrons. See the Example Below for Two Isotopes of Oxygen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Strontium-90
EPA Facts about Strontium-90 What is strontium-90? The most common isotope of strontium is strontium-90. Radioactive strontium-90 is produced when uranium and plutonium undergo fission. Fission The time required for a radioactive substance to is the process in which the nucleus of a lose 50 percent of its radioactivity by decay is radionuclide breaks into smaller parts. Large known as the half-life. Strontium-90 has a half- amounts of radioactive strontium-90 were life of 29 years and emits beta particles of produced during atmospheric nuclear weapons relatively low energy as it decays. Yttrium-90, its tests conducted in the 1950s and 1960s. As a decay product, has a shorter half-life (64 hours) result of atmospheric testing and radioactive than strontium-90, but it emits beta particles of fallout, this strontium was dispersed and higher energy. deposited on the earth. How are people exposed to strontium- 90? What are the uses of strontium-90? Although external exposure to strontium-90 Strontium-90 is used in medical and agricultural from nuclear testing is of minor concern because studies. It is also used in thermoelectric devices environmental concentrations are low, that are built into small power supplies for use strontium in the environment can become part of the food chain. This pathway of exposure in remote locations, such as navigational beacons, remote weather stations, and space became a concern in the 1950s with the advent vehicles. Additionally, strontium-90 is used in of atmospheric testing of nuclear explosives. electron tubes, radioluminescent markers, as a With the suspension of atmospheric testing of radiation source in industrial thickness gauges, nuclear weapons, dietary intake has steadily and for treatment of eye diseases. -

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

The Chemistry of Strontium and Barium Scales
Association of Water Technologies October 20 -23, 2010 Reno, NV, USA The Chemistry of Strontium and Barium Scales Robert J. Ferguson and Baron R. Ferguson French Creek Software, Inc. Kimberton, PA 19442 (610) 935-8337 (610) 935-1008 FAX [email protected] [email protected] Abstract New ‘mystery scales’ are being encountered as cooling tower operators increase cycles to new highs, add ‘reuse’ water to the make-up, and utilize new make-up water sources as part of an overall water conservation strategy. Scales rarely, if ever, encountered in the past are emerging as potential problems. This threat of unexpected scale is compounded because most water treatment service companies do not include barium and strontium in their make-up water analyses. Water sources with even as little 0.01 mg/L of Ba (as Ba) can become very scale-forming with respect to barite (BaSO4) when tower concentration ratios are increased and sulfuric acid used for pH control. Make-up waters incorporating reverse osmosis concentrate can also provide a strontium and barium source. In some cases, produced waters are also being used in an effort for greener water use. This paper discusses the chemistry of the barium and strontium based scales barite (BaSO4), celestite (SrSO4), witherite (BaCO3) and strontianite (SrCO3). Conditions for formation and control from a water treater’s perspective are emphasized. Indices for prediction are discussed. Scale Prediction and the Concept of Saturation A majority of the indices used routinely by water treatment chemists are derived from the basic concept of saturation. A water is said to be saturated with a compound (e.g. -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -

4. CHEMICAL, PHYSICAL, and RADIOLOGICAL INFORMATION
STRONTIUM 189 4. CHEMICAL, PHYSICAL, and RADIOLOGICAL INFORMATION 4.1 CHEMICAL IDENTITY Strontium is an alkaline earth element in Group IIA of the periodic table. Because of its high reactivity, elemental (or metallic) strontium is not found in nature; it exists only as molecular compounds with other elements. The chemical information for elemental strontium and some of its compounds is listed in Table 4-1. Radioactive isotopes of strontium (e.g., 89Sr and 90Sr, see Section 4.2) are the primary cause of concern with regard to human health (see Chapter 3). 4.2 PHYSICAL, CHEMICAL, AND RADIOLOGICAL PROPERTIES The physical properties of strontium metal and selected strontium compounds are listed in Table 4-2. The percent occurrence of strontium isotopes and the radiologic properties of strontium isotopes are listed in Table 4-3. Strontium can exist in two oxidation states: 0 and +2. Under normal environmental conditions, only the +2 oxidation state is stable enough to be of practical importance since it readily reacts with both water and oxygen (Cotton and Wilkenson 1980; Hibbins 1997). There are 26 isotopes of strontium, 4 of which occur naturally. The four stable isotopes, 84Sr, 86Sr, 87Sr, and 88Sr, are sometimes referred to as stable strontium. The most important radioactive isotopes, 89Sr and 90Sr, are formed during nuclear reactor operations and during nuclear explosions by the nuclear fission of 235U, 238U, or 239Pu. For example, 235U is split into smaller atomic mass fragments such as 90Sr by a nuclear chain reaction initiated by high energy neutrons of approximately 1 million electron volts (or 1 MeV). -
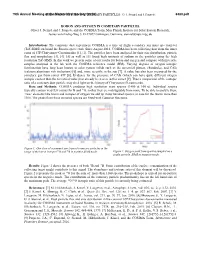
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u). -

Investigation of Nitrogen Cycling Using Stable Nitrogen and Oxygen Isotopes in Narragansett Bay, Ri
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2014 INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI Courtney Elizabeth Schmidt University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Schmidt, Courtney Elizabeth, "INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI" (2014). Open Access Dissertations. Paper 209. https://digitalcommons.uri.edu/oa_diss/209 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI BY COURTNEY ELIZABETH SCHMIDT A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN OCEANOGRAPHY UNIVERSITY OF RHODE ISLAND 2014 DOCTOR OF PHILOSOPHY DISSERTATION OF COURTNEY ELIZABETH SCHMIDT APPROVED: Thesis Committee: Major Professor Rebecca Robinson Candace Oviatt Arthur Gold Nassar H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT Estuaries regulate nitrogen (N) fluxes transported from land to the open ocean through uptake and denitrification. In Narragansett Bay, anthropogenic N loading has increased over the last century with evidence for eutrophication in some regions of Narragansett Bay. Increased concerns over eutrophication prompted upgrades at wastewater treatment facilities (WWTFs) to decrease the amount of nitrogen discharged. The upgrade to tertiary treatment – where bioavailable nitrogen is reduced and removed through denitrification – has occurred at multiple facilities throughout Narragansett Bay’s watershed. -

Strontium-90 and Strontium-89: a Review of Measurement Techniques in Environmental Media
STRONTIUM-90 AND STRONTIUM-89: A REVIEW OF MEASUREMENT TECHNIQUES IN ENVIRONMENTAL MEDIA Robert J. Budnitz Lawrence Berkeley Laboratory University of California Berkeley, CA 91*720 1. IntroiUiction 2. Sources of Enivronmental Radiostrontium 3. Measurement Considerations a. Introduction b. Counting c. •»Sr/"Sr Separation 4. Chemical Techniques a. Air b. Water c. Milk d. Other Media e. Yttrium Recovery after Ingrowth f. Interferences S- Calibration Techniques h. Quality Control 5. Summary and Conclusions 6. Acknowli'J.jnent 7. References -NOTICE- This report was prepared as an account of worK sponsored by the United States Government. Neither the United States nor the United Slates Atomic Energy Commission, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any wananty, express or implied, or asjumes any legal liabili:y or responsibility for the accuracy, com pleteness or usefulness of any Information, apparatus, J product or process disclosed, or represents that its use I would not infringe privately owned rights. Mmh li\ -1- 1. INTRODUCTION There are only two radioactive isotopes SrflO of strontium of significance in radiological measurements in the environment: strontium-89 Avg.jS energy 196-1 k«V and strontium-90. 100 Strontium-90 is the more significant from the point of view of environmental impact. 80 Energy keV 644 This is due mostly to its long half-life % Emission 100 (28.1 years). It is a pure beta emitter with 60 Typ* of only one decay mode, leading to yttrium-90 •mission by emission of a negative beta with HUBX * 40 h S46 keV. -

PUBLIC HEALTH STATEMENT Strontium CAS#: 7440-24-6
PUBLIC HEALTH STATEMENT Strontium CAS#: 7440-24-6 Division of Toxicology April 2004 This Public Health Statement is the summary External exposure to radiation may occur from chapter from the Toxicological Profile for natural or man-made sources. Naturally occurring strontium. It is one in a series of Public Health sources of radiation are cosmic radiation from space Statements about hazardous substances and their or radioactive materials in soil or building materials. health effects. A shorter version, the ToxFAQs™, is Man-made sources of radioactive materials are also available. This information is important found in consumer products, industrial equipment, because this substance may harm you. The effects atom bomb fallout, and to a smaller extent from of exposure to any hazardous substance depend on hospital waste and nuclear reactors. the dose, the duration, how you are exposed, personal traits and habits, and whether other If you are exposed to strontium, many factors chemicals are present. For more information, call determine whether you’ll be harmed. These factors the ATSDR Information Center at 1-888-422-8737. include the dose (how much), the duration (how _____________________________________ long), and how you come in contact with it. You must also consider the other chemicals you’re This public health statement tells you about exposed to and your age, sex, diet, family traits, strontium and the effects of exposure. lifestyle, and state of health. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in 1.1 WHAT IS STRONTIUM? the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for Strontium is a natural and commonly occurring long-term federal cleanup activities. -

Sequential Isotopic Determination of Plutonium, Strontium, Americium
Sequential Isotopic Determination of Plutonium, Americium, Uranium, and Strontium in Soil Sample Jeng-Jong Wang, Shing-Fa Fang, and Tzu-Wen Wang Institute of Nuclear Energy Research, Atomic Energy Council, Taiwan, R.O.C. Abstract A procedure is developed to provide sequential analysis of 238Pu, 239/240Pu, 241Am, 238U, and 90Sr in soil sample. Tracers and/or carriers (242Pu, 243Am, 232U, and stable strontium) are added into the soil sample as chemical yield monitors, and then digested and extracted with nitric acid. Plutonium, strontium, americium, and uranium are sequential separated and purified by Dowex ion-exchange resin, EiChroM Sr-resin, EiChroM TRU-resin, and Chelate-100 resin, respectively. 90Sr is measured by Cerenkov counting. The actinides are determined by alpha-particle spectrometer. This method is verified by analyzed the U.S. National Institute of Standard and Technology NRIP2000 soil samples. The analytical results of 241Am, 238Pu, 239/240Pu, 238U, and 90Sr agree with the NIST values within +5.7%, +2.4%, +1.6%, -2.2%, and -2.7%, respectively. According to the traceability limits defined in ANSI N42.22 criteria and measurement traceability to NIST for low-level radionuclides has been demonstrated to be better than 23%, 22%, 22%, 22% and 34% for 241Am, 238Pu, 239/240Pu, 238U, and 90Sr, respectively. Keywords: sequential analysis, NIST, traceability Introduction 238Pu, 239/240Pu, 241Am, 238U, and 90Sr in the environmental samples, such as soil, vegetation, and water, are frequently analyzed for both emergency and routine radiation monitoring. Health physicists use the analytical results of environmental samples to estimate the amount of radioactive material present in the environment, calculating its burden for the radiological workers and the general public. -

Analytical Developments in the Measurements of Boron, Nitrate
XA9848324 ANALYTICAL DEVELOPMENTS IN THE MEASUREMENTS OF BORON, NITRATE, PHOSPHATE AND SULPHATE ISOTOPES AND CASE EXAMPLES OF DISCRIMINATION OF NITROGEN AND SULPHUR SOURCES IN POLLUTION STUDIES J. AGGARWAL, D.S. SHEPPARD Institute of Geological & Nuclear Sciences, Lower Hutt B.W. ROBINSON Wairakei Research Centre, Institute of Geological & Nuclear Sciences, Taupo New Zealand Abstract - Methods are documented for the analysis of B isotopes, O and N isotopes in nitrates. B isotopes can be measured by negative ion thermal ionisation mass spectrometry. Nitrate is recovered from groundwaters by ion exchange and the resulting silver nitrate combusted for stable isotope gas analysis. Oxygen isotope analysis of phosphates can be determined by generating and analysing CO2 gas from the combustion of silver phosphate produced from aqueous samples. Sulphate in ground and surface waters can be separated and concentrated by ion exchange and precipitated as barium sulphate. This is reacted with graphite to yield CO2 and CO, the latter being spark discharged to CO2 and the total CO2 measured for oxygen isotope analysis. Barium sulphide from this reaction is converted to silver sulphide which is reacted with cuprous oxide to give SO2 gas for sulphur isotope measurements. A case study of the semi-rural Manakau area in New Zealand was conducted to see if nitrate isotopes could be used to detect the source of nitrate contamination (groundwater nitrate <0.5 to 11.3 mg/L NO'3-N). Nitrogen isotope (+4 to +12%o) coupled with oxygen isotope measurements (+5 to +9%o) demonstrated that the nitrogen is not sourced from fertilisers but from some combination of septic tank and animal waste. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table.