Quality Measure Tip Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Mitomycin) for Pyelocalyceal Solution with a Nephrostomy Tube
INSTRUCTION GUIDE for Instillation of JELMYTO® (mitomycin) for pyelocalyceal solution with a Nephrostomy Tube Important Information Please refer to the FDA-approved Prescribing Information and Instructions for Administration (IFA) for information about JELMYTO. JELMYTO is a cytotoxic drug. Follow applicable special handling and disposal procedures. This Instruction Guide contains supplemental information on how to instill JELMYTO via a nephrostomy tube. These instructions are derived from materials from the OLYMPUS Clinical Trial. It is important to note that in the OLYMPUS Clinical Trial, no investigators utilized a nephrostomy tube as the mode of administration. Important Information Regarding Nephrostomy Instillation Note: Follow practice/institutional protocol regarding placement for nephrostomy tube and time allowance for healing before proceeding with JELMYTO instillation. • Prior to administration, implement your clinic's practice for draining the patients' urine. • A Silicon or Silastic percutaneous nephrostomy tube with molded Luer lock connector and locking loop must be placed prior to installation of JELMYTO. Latex should not be used, due to risk of rupture. Instillation Instructions A. Measure the Kidney Volume Before the First Instillation The Instillation Volume will be equal to the patient’s kidney volume. If the kidney volume is already known, proceed to Section B. If the kidney volume is NOT known, or needs to be reassessed, use the following steps: 1. Perform an antegrade pyelogram using diluted contrast (50%) so that the entire renal pelvis and calyces are observed and contrast starts to flow below the ureteropelvic junction (UPJ). 2. Record the volume of contrast injected at this point. 3. Allow the contrast to drain from the kidney. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
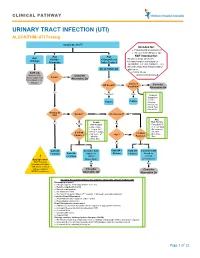
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

Obstructive Nephropathy Saulo Klahr
REVIEW ARTICLE Obstructive Nephropathy Saulo Klahr Abstract ages. The incidence of hydronephrosis reported by Bell (1) in a series of32,360 autopsies was 3.8% (3.9% in males, 3.6% in Obstructive nephropathy is a relatively commonentity females). The incidence of clinical manifestations of obstruc- that is treatable and often reversible. It occurs at all ages tive uropathy prior to death was not reported, and it is likely from infancy to elderly subjects. Obstructive uropathy is that hydronephrosis was an incidental finding in many of these classified according to the degree, duration and site of the patients. The incidence of hydronephrosis at autopsy is some- obstruction. It is the result of functional or anatomic le- what lower in children than in adults, being 2%in one series of sions located in the urinary tract. The causes of obstructive 16, 100 autopsies (2). Over 80% of children with hydronephro- uropathy are many. Obstruction of the urinary tract may sis at autopsy were less than 1 year old, with the balance of decrease renal blood flow and the glomerular filtration rate. childhood cases being distributed uniformly through the child- Several abnormalities in tubular function mayoccur in hood years. About 166 patients per 100,000 population had a obstructive nephropathy. These include decreased reab- presumptive diagnosis of obstructive uropathy on admission sorption of solutes and water, inability to concentrate the to hospitals in the United States in 1985 (3). Amongmale pa- urine and impaired excretion of hydrogen and potassium. tients with kidney and urologic disorders, obstructive uropa- Renal interstitial fibrosis is a commonfinding in patients thy ranked fourth at discharge (242 patients/100,000 dis- with long-term obstructive uropathy. -

Percutaneous Nephrostomy
Page 1 of 6 Percutaneous Nephrostomy (PCN) Tube Related Infections Disclaimer: This algorithm has been developed for MD Anderson using a multidisciplinary approach considering circumstances particular to MD Anderson’s specific patient population, services and structure, and clinical information. This is not intended to replace the independent medical or professional judgment of physicians or other health care providers in the context of individual clinical circumstances to determine a patient's care. This algorithm should not be used to treat pregnant women. Local microbiology and susceptibility/resistance patterns should be taken into consideration when selecting antibiotics. CLINICAL EVALUATION PRESENTATION Patient presentation suspicious for PCN infection: ● Lower UTI: dysuria, frequency, urgency and/or suprapubic pain ● Upper UTI: fever/chills, leukocytosis and/or CVA tenderness (with or without lower UTI symptoms) ● PCN exit site infection: local signs for erythema, ● Renal ultrasound or CT abdomen and pelvis with contrast warmth, tenderness and/or purulence ● Consider Infectious Diseases (ID) consult ● See Sepsis Management - Adult algorithm Yes Labs: Does ● CBC with differential, patient exhibit at BUN, creatinine least two modified ● Blood cultures SIRS criteria2 and ● Urine analysis and urine 1 hemodynamic ● Start empiric IV antibiotics (refer to Page 2) culture from urethral 3 instability ? ● Consider CT abdomen and pelvis with and PCN sites No contrast to rule out hydronephrosis, Fever, chills, Yes pyelonephritis and renal -

Primary Obstructive Megaureter in a Child: a Case Report and Review of Literature
Case Report Annals of Pediatric Research Published: 10 May, 2019 Primary Obstructive Megaureter in a Child: A Case Report and Review of Literature Volkan Sarper Erikci* and Tunahan Altundag Department of Pediatric Surgery, Tepecik Training Hospital, Turkey Abstract Ureterovesical Junction Obstruction (UVJO) is a rare but important cause of hydroureteronephrosis in childhood. A 2-years-old boy suffered from giant hydroureteronephrosis originating from idiopathic ureterovesical junction obstruction. He was treated with excision of narrow ureteric segment with tapering ureteroplasty and a ureteral reimplantation was performed. This case is presented and discussed with reference to etiology of this rather rare anomaly. Keywords: Hydroureteronephrosis; Ureterovesical junction obstruction; Children Introduction Congenital Ureterovesical Junction Obstruction (UVJO) may be observed during fetal age or any stage at the time of childhood. It is aimed in this report to present a male child with obstructive megaureter due to congenital UVJO. He was surgically treated with excision of narrowed distal ureter in addition to tapering ureteroplasty with ureteroneocystostomy. The topic is discussed with special reference to the etiology of this rather rare entity under the light of relevant literature. Case Presentation A 27-months-old boy was admitted to our department with an antenatal history of right Hydroureteronephrosis (HUN). Laboratory tests were otherwise normal except signs of Urinary OPEN ACCESS Tract Infection (UTI) including leucocyturia. -

The Presentation and Management of Neonatal Obstructive Uropathies J
Postgrad Med J: first published as 10.1136/pgmj.48.562.486 on 1 August 1972. Downloaded from Postgraduate Medical Journal (August 1972) 48, 486 -492. The presentation and management of neonatal obstructive uropathies J. H. JOHNSTON F.R.C.S. Alder Hey Children's Hospital, Liverpool Presentation urinary, alimentary and genital tracts share a The existence of a congenital urinary obstruction common evacuatory channel is similarly frequently may be suggested when routine examination of the associated with upper urinary tract lesions. newborn infant reveals such signs as enlargement of one or both kidneys, distension of the bladder or (3) Anomalies of the female genital tract slow, dribbling micturition. The paediatrician should Congenital absence of the vagina is associated also be alerted to the possibility of obstructive uro- with urinary tract anomalies in some 500 of cases pathy when there are present non-urological con- (Bryan, Nigro & Counsellor, 1949). A similar high genital anomalies which often secondarily involve incidence occurs with such conditions as duplication the urinary tract or which are known commonly to ofthe vagina and uterus but these may not be obvious co-exist with congenital urinary tract lesions. clinically in the young child. Secondary involvement, with obstruction, of the urinary tract occurs with space-occupying masses in Deficient abdominal mucsculature (4) by copyright. the pelvis such as hydrometrocolpos or a large intra- Urinary tract anomalies of various degrees of pelvic component of a sacrococcygeal teratoma. are The pelvic tumour, by displacing the bladder, leads severity always present. to chronic urinary retention and upper tract dilata- Cardiac anomalies tion. -

Percutaneous Nephrostomy
SEMINARS IN INTERVENTIONAL RADIOLOGY VOLUME 17, NUMBER 4 2000 Percutaneous Nephrostomy Michael M. Maher, M.D., Timothy Fotheringham, M.D., and Michael J. Lee, M.D. ABSTRACT Percutaneous nephrostomy (PCN) is now established as a safe and effective early treatment for patients with urinary tract obstruction. In experienced hands, PCN is usually successful and is associated with low complication rates. This article discusses all aspects of PCN, including the indications and contraindications to PCN, patient preparation, the techniques employed for gaining access to the urinary tract, and placement of the PCN tube. In addition, we discuss the complications of PCN and possible ways of avoiding them, as well as the care of patients following PCN. Keywords: Percutaneous nephrostomy, renal failure, pyonephrosis Urinary tract obstruction invariably requires eral anesthesia is avoided and surrounding organs treatment, and indications for immediate or urgent can be imaged and avoided during PCN. In expert intervention include clinical and laboratory signs of hands, PCN is associated with a major complication underlying sepsis and clinical or radiological signs rate of 4 to 5%4 and a minor complication rate of of pyonephrosis. Urgent intervention is also manda- approximately 15%.1 PCN is usually faster, less ex- tory for the treatment of life-threatening biochemi- pensive, and less likely to be associated with compli- cal abnormalities associated with the rapid onset of cations than is surgery.5 In the current era, PCN renal failure. also allows access to the urinary tract for more In the past decade, image-guided PCN has re- definitive treatment of underlying pathologies such placed the conventional surgical approach for the as percutaneous nephrolithotomy (PCNL) for temporary drainage of urinary tract obstruction. -

Nephrostomy Tubes
Charlotte Derr, MD, FACEP Tubes and Lines Clogged or Broken: Trouble Shooting Tubes and Lines Charlotte Derr, MD, FACEP Associate Program Director • Director of Emergency Ultrasound University of South Florida Emergency Medicine Residency Program Central Venous Catheters Types of Central Venous Catheters (CVC) Tunneled o Used for medium or long-term access requirements . Chemotherapy . TPN . Hemodialysis o Single, double, or triple lumen o Usually inserted via access to a neck vein o May be placed percutaneously by interventional radiology (IR) or surgically o The catheter is tunneled through the adjacent subcutaneous tissues to the vein then exits the skin over the chest wall o Likely provides a barrier to ascending infection o Will adhere to subcutaneous tissues over time o Less chance for dislodgement but removal may be difficult o Examples . Hickman, Groshong, Broviac catheters . Totally Implanted Venous Access Devices (iVADs or “portacath”) Has a reservoir attached to the catheter that is buried in the subcutaneous tissue of the chest wall Accessed percutaneously via a noncoring needle Can be accessed up to 1,000 times Has lower infection rates than external catheters Best for intermittent therapies (e.g. factor or enzyme infusions) Tolerates immersion for bathing Non-tunneled o Placed directly into the vein via a skin incision or puncture overlying the vein o Placed in the neck, upper chest, extremities o Examples . Triple lumen polyurethane catheters, percutaneous sheath introducer kits Shorter-term therapies, such as during hospitalization 1 Charlotte Derr, MD, FACEP Tubes and Lines Indications o Extended antibiotic treatment o Repeated blood sampling o Vasopressors o TPN o Hemodynamic monitoring (pulmonary artery catheters) . -

Suprapubic Cystostomy and Nephrostomy Care This Brochure Will Help You Learn How to Care for Your Catheter
Northwestern Memorial Hospital Patient Education CARE AND TREATMENT Suprapubic Cystostomy and Nephrostomy Care This brochure will help you learn how to care for your catheter. The following are general guidelines. If you have any questions or concerns, please ask your physician or nurse. Wash your A suprapubic cystostomy is a surgical Figure 1. Suprapubic hands carefully opening made into the bladder directly cystostomy above the pubic bone. A tube (catheter) before and is inserted into the bladder. The catheter is held in place by a balloon or sutures. after changing Urine flows through the catheter into a Catheter drainage bag (Figure 1). the bandage or A nephrostomy tube works much the same way, except: Tape drainage bag. ■ The surgical opening is made into the kidney. Drainage bag ■ The catheter is held in place by sutures and/or a wax wafer with a catheter holder (Figure 2). Figure 2. Nephrostomy General guidelines It is important to keep the area around the catheter site clean. Change the gauze bandage every day or any time it comes off. Change the catheter tape when it becomes soiled or loose. When taping the catheter to your skin, make sure the catheter is not kinked. If your skin is sensitive to adhesive bandage tape, use a Catheter non-allergenic tape. Wash your hands carefully before and after changing the bandage Tape or drainage bags. Avoid pulling on the catheter or tube. Do not clamp your catheter or tube. You may notice dried crusts around the outside of the catheter. These can be removed by gently wiping with a wet washcloth. -

Acute Necrotizing Ureteritis with Obstructive Uropathy Following Instillation of Silver Nitrate in Chyluria: a Case Report
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector C.M. Su, Y.C. Lee, W.J. Wu, et al ACUTE NECROTIZING URETERITIS WITH OBSTRUCTIVE UROPATHY FOLLOWING INSTILLATION OF SILVER NITRATE IN CHYLURIA: A CASE REPORT Chin-Ming Su, Yung-Chin Lee, Wen-Jen Wu, Hung-Lung Ke, Yii-Her Chou, and Chun-Hsiung Huang Department of Urology, Kaohsiung Medical University, Kaohsiung, Taiwan. Chyluria occurs as a result of communication between the lymphatics and the renal pelvis. It is believed that instillation of silver nitrate into the renal pelvis is a safe, minimally invasive and effective treatment for chyluria. We report an unusual complication of acute necrotizing ureteritis following instillation of silver nitrate in a case of chyluria. It resolved completely with non-surgical intervention. The diagnosis and management of chyluria is discussed, with a brief review of the literature. Key Words: chyluria, silver nitrate instillation, necrotizing ureteritis (Kaohsiung J Med Sci 2004;20:512–5) Chyluria occurs as a result of communication between the radiation, or parasite infection were known. The patient lymphatics and the renal pelvis. Its etiology may be classi- underwent cystoscopic examination following a fatty fied as parasitic or non-parasitic. Although the disease is meal. A milky efflux was observed from the right ureteral not life-threatening, it may cause hypoproteinemia, weight orifice. Retrograde pyelography was normal, with no loss, and immunologic disorders due to severe proteinuria pyelolymphatic backflow (Figure 1). Under the diagnosis of [1]. The treatment of chyluria includes limitation of diet to chyluria, 10 mL of 1% silver nitrate was instilled through a mid-chain triglycerides, instillation of silver nitrate, and ureteric catheter.