Project Report No. RD-2006-3293/ LK0991 New Environmentally
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACTX-Hv1c, Isolated from the Venom of the Blue Mountains
S.J. Gunning et al. Janus-faced atracotoxins block KCa channels The Janus-faced atracotoxins are specific blockers of invertebrate KCa channels Simon J. Gunning1, Francesco J. Maggio2,4, Monique J. Windley1, Stella M. Valenzuela1, Glenn F. King3 and Graham M. Nicholson1 1 Neurotoxin Research Group, Department of Medical & Molecular Biosciences, University of Technology, Sydney, Broadway, NSW 2007, Australia 2 Department of Molecular, Microbial & Structural Biology, University of Connecticut School of Medicine, Farmington, Connecticut 06032, USA and 3 Division of Chemical and Structural Biology, Institute for Molecular Bioscience, University of Queensland, Brisbane QLD 4072, Australia 4 Current affiliation: Bristol-Myers Squibb, 6000 Thompson Road, Syracuse NY 13057, USA Subdivision: Molecular Neurobiology Abbreviations: ACTX, atracotoxin; 4-AP, 4-aminopyridine; BKCa channel, 2+ + 2+ large-conductance Ca -activated K channel; CaV channel, voltage-activated Ca channel; ChTx, charybdotoxin; DRG, dorsal root ganglia; DUM, dorsal unpaired median; EGTA, ethylene glycol-bis(b-aminoethyl ether)-N,N,N’N’-tetraacetic acid; IbTx, + iberiotoxin; J-ACTX, Janus-faced atracotoxin; KA channel, transient ‘A-type’ K channel; 2+ + + KCa channel, Ca -activated K channel; KDR channel, delayed-rectifier K channel; KV + + channel, voltage-activated K channel; NaV channel, voltage-activated Na channel; Slo, Slowpoke; TEA, tetraethylammonium; TTX, tetrodotoxin. RUNNING TITLE: Janus-faced atracotoxins block KCa channels ADDRESS CORRESPONDENCE TO: Graham M. Nicholson, Department of Medical & Molecular Biosciences, University of Technology, Sydney, PO Box 123, Broadway NSW 2007, Australia. Tel: +61 2 9514-2230 Fax: +61 2 9514-2228; E-mail: [email protected] 1 S.J. Gunning et al. Janus-faced atracotoxins block KCa channels ABSTRACT The Janus-faced atracotoxins are a unique family of excitatory peptide toxins that contain a rare vicinal disulfide bridge. -

Versatile Spider Venom Peptides and Their Medical and Agricultural Applications
Accepted Manuscript Versatile spider venom peptides and their medical and agricultural applications Natalie J. Saez, Volker Herzig PII: S0041-0101(18)31019-5 DOI: https://doi.org/10.1016/j.toxicon.2018.11.298 Reference: TOXCON 6024 To appear in: Toxicon Received Date: 2 May 2018 Revised Date: 12 November 2018 Accepted Date: 14 November 2018 Please cite this article as: Saez, N.J., Herzig, V., Versatile spider venom peptides and their medical and agricultural applications, Toxicon (2019), doi: https://doi.org/10.1016/j.toxicon.2018.11.298. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT MANUSCRIPT ACCEPTED ACCEPTED MANUSCRIPT 1 Versatile spider venom peptides and their medical and agricultural applications 2 3 Natalie J. Saez 1, #, *, Volker Herzig 1, #, * 4 5 1 Institute for Molecular Bioscience, The University of Queensland, St. Lucia QLD 4072, Australia 6 7 # joint first author 8 9 *Address correspondence to: 10 Dr Natalie Saez, Institute for Molecular Bioscience, The University of Queensland, St. Lucia QLD 11 4072, Australia; Phone: +61 7 3346 2011, Fax: +61 7 3346 2101, Email: [email protected] 12 Dr Volker Herzig, Institute for Molecular Bioscience, The University of Queensland, St. -

CSTX-1, a Toxin from the Venom of the Hunting Spider Cupiennius Salei, Is
For submission to Neuropharmacology CSTX-1, a toxin from the venom of the hunting spider Cupiennius salei, is a selective blocker of L-type calcium channels in mammalian neurons *,1Helmut Kubista, 2Roberta A. Mafra, 3Youmie Chong, 3Graham M. Nicholson, 2Paulo S.L. Beirão, 2Jader S. Cruz, 4Stefan Boehm, 5Wolfgang Nentwig & 5Lucia Kuhn-Nentwig 1Center for Biomolecular Medicine and Pharmacology, Institute of Pharmacology, Medical University of Vienna, Waehringerstrasse 13a, A-1090, Vienna, Austria 2Department of Biochemistry and Immunology, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Av. Antonio Carlos 6627, 31270-901 Belo Horizonte, MG, Brazil 3Neurotoxin Research Group, Department of Medical & Molecular Biosciences, University of Technology, Sydney, City Campus, Broadway, Sydney, NSW 2007, Australia 4Institute of Experimental and Clinical Pharmacology, Medical University of Graz, Universitätsplatz 4, A-8010 Graz, Austria 5Zoological Institute, University of Bern, Baltzerstrasse 6, CH-3012 Bern, Switzerland *Author for correspondence: Telephone: ++43 1 4277 64146 Fax: ++ 43 1 4277 9641 E-mail: [email protected] H. Kubista et al. CSTX-1, a selective Cav channel blocker 2 Abstract The inhibitor cystine-knot motif identified in the structure of CSTX-1 from Cupiennius salei venom suggests that this toxin may act as a blocker of ion channels. Whole-cell patch clamp experiments performed on cockroach neurons revealed that CSTX-1 produced a slow voltage- independent block of both mid/low- (M-LVA) and high-voltage-activated (HVA) insect Cav channels. Since Cupiennius salei venom affects both insect as well as rodent species, we investigated whether Cav channel currents of rat neurons are also inhibited by CSTX-1. -

Structure-Function Studies of Insecticidal Atracotoxins
STRUCTURE-FUNCTION STUDIES OF INSECTICIDAL ATRACOTOXINS PhD Thesis SIMON GUNNING UTS 2009 II ABSTRACT Part I The -atracotoxins (-ACTXs, previously the Janus-faced atracotoxins ) are a family of five insect-selective excitatory peptide neurotoxins containing 36-37 residues with four disulfide bonds. Toxins from this family were isolated from the venom of the Blue Mountains funnel-web spider (Hadronyche versuta) and Toowooba funnel-web spider (Hadronyche infensa). The NMR solution structure and primary sequence of the prototypic member -ATCT-Hv1c provided few clues as to the likely molecular target. In order to characterise the site of action and phylogenetic specificity of these toxins, whole-cell patch-clamp electrophysiology was employed using isolated DUM neurons from the American cockroach (Periplaneta americana). -ACTX-Hv1c had no effect on the gating or kinetics of INa or ICa at concentrations up to 1 M. However, at the same concentration, -ATCT-Hv1c reduced Kv channel currents by 56 ± 7% (n = 5). Subsequent experiments in insect DUM neurons indicated that inhibition of the macroscopic IK was due to a block of calcium-activated Kv (KCa) channels, with an IC50 of 2.3 nM and 2.9 nM for peak and late IK(Ca) respectively (n = 5), and not ‘A-type’ or delayed-rectifier Kv channels. Insect selectivity was confirmed by a lack of activity on rat dorsal root ganglion (DRG) neuron global IK as well as IK(Ca) at doses up to 1 µM. -ACTX-Hv1c is a selective insect KCa (BKCa) channel pore- blocker, not a gating modifier, as inhibition of insect IK(Ca) occurred in the absence of any voltage-dependent actions on channel activation. -

Origin and Characterization of Extracellular Vesicles Present in the Spider Venom of Ornithoctonus Hainana
toxins Article Origin and Characterization of Extracellular Vesicles Present in the Spider Venom of Ornithoctonus hainana Chengfeng Xun, Lu Wang, Hailin Yang, Zixuan Xiao, Min Deng, Rongfang Xu , Xi Zhou , Ping Chen * and Zhonghua Liu * The National and Local Joint Engineering Laboratory of Animal Peptide Drug Development, College of Life Sciences, Hunan Normal University, Changsha 410081, China; [email protected] (C.X.); [email protected] (L.W.); [email protected] (H.Y.); [email protected] (Z.X.); [email protected] (M.D.); [email protected] (R.X.); [email protected] (X.Z.) * Correspondence: [email protected] (P.C.); [email protected] (Z.L.) Abstract: Extracellular vesicles (EVs), including exosomes and microvesicles, are membranous vesicles released from nearly all cellular types. They contain various bioactive molecules, and their molecular composition varies depending on their cellular origin. As research into venomous animals has progressed, EVs have been discovered in the venom of snakes and parasitic wasps. Although vesicle secretion in spider venom glands has been observed, these secretory vesicles’ origin and biological properties are unknown. In this study, the origin of the EVs from Ornithoctonus hainana venom was observed using transmission electron microscopy (TEM). The Ornithoctonus hainana venom extracellular vesicles (HN-EVs) were isolated and purified by density gradient centrifugation. HN-EVs possess classic membranous vesicles with a size distribution ranging from 50 to 150 nm and express the arthropod EV marker Tsp29Fb. The LC-MS/MS analysis identified a total of 150 proteins, which were divided into three groups according to their potential function: conservative vesicle transport-related proteins, virulence-related proteins, and other proteins of unknown function. -
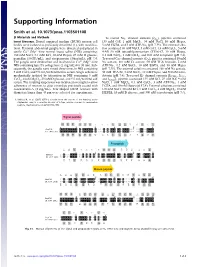
Supporting Information
Supporting Information Smith et al. 10.1073/pnas.1103501108 SI Materials and Methods I To record NaV channel currents ( Na), pipettes contained Insect Bioassays. Dorsal unpaired median (DUM) neuron cell 135 mM CsF, 1 mM MgCl2, 34 mM NaCl, 10 mM Hepes, bodies were isolated as previously described (1), with modifica- 5 mM EGTA, and 3 mM ATP-Na2 (pH 7.35). The external solu- tions. Terminal abdominal ganglia were dissected and placed in tion contained 80 mM NaCl, 5 mM CsCl, 1.8 mM CaCl2,5mM sterile Ca2þ∕Mg2þ-free normal insect saline (NIS) containing: 4-AP, 50 mM tetraethlyammonium (TEA)-Cl, 10 mM Hepes, 180 mM NaCl, 3.1 mM KCl, 10 mM Hepes, 25 mM D glucose, 0.1 mM NiCl2, 1 mM CdCl2, and 0.01 mM verapamil (pH 7.4). 50 ∕ 50μ ∕ I penicillin ( IU mL), and streptomycin ( g mL), pH 7.4. To record CaV channel currents ( Ca), pipettes contained 10 mM The ganglia were desheathed and incubated in Ca2þ∕Mg2þ-free Na acetate, 110 mM Cs acetate, 50 mM TEA bromide, 2 mM NIS containing type IA collagenase (2 mg∕mL) for 38 min. Sub- ATP-Na2, 0.5 mM BaCl2, 10 mM EGTA, and 10 mM Hepes sequently, the ganglia were rinsed three times in NIS containing (pH 7.35). The external solution contained 160 mM Na acetate, 5 mM CaCl2 and 5% vol∕vol fetal bovine serum. Single cells were 30 mM TEA Br, 3 mM BaCl2, 10 mM Hepes, and 500 nM tetro- K I I mechanically isolated by trituration in NIS containing 5 mM dotoxin (pH 7.4). -
![Toxins Review Oglsigfsiaino Uastwrsvnmu Nml [5]](https://docslib.b-cdn.net/cover/5918/toxins-review-oglsigfsiaino-uastwrsvnmu-nml-5-6665918.webp)
Toxins Review Oglsigfsiaino Uastwrsvnmu Nml [5]
toxins Review Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses Nicolas Langenegger *, Wolfgang Nentwig and Lucia Kuhn-Nentwig * Institute of Ecology and Evolution, University of Bern, Baltzerstrasse 6, CH-3012 Bern, Switzerland; [email protected] * Correspondence: [email protected] (N.L.); [email protected] (L.K.-N.) Received: 26 September 2019; Accepted: 18 October 2019; Published: 22 October 2019 Abstract: This review gives an overview on the development of research on spider venoms with a focus on structure and function of venom components and techniques of analysis. Major venom component groups are small molecular mass compounds, antimicrobial (also called cytolytic, or cationic) peptides (only in some spider families), cysteine-rich (neurotoxic) peptides, and enzymes and proteins. Cysteine-rich peptides are reviewed with respect to various structural motifs, their targets (ion channels, membrane receptors), nomenclature, and molecular binding. We further describe the latest findings concerning the maturation of antimicrobial, and cysteine-rich peptides that are in most known cases expressed as propeptide-containing precursors. Today, venom research, increasingly employs transcriptomic and mass spectrometric techniques. Pros and cons of venom gland transcriptome analysis with Sanger, 454, and Illumina sequencing are discussed and an overview on so far published transcriptome studies is given. In this respect, we also discuss the only recently -
Neurotoxin Merging: a Strategy Deployed by the Venom of the Spider Cupiennius Salei to Potentiate Toxicity on Insects
toxins Article Neurotoxin Merging: A Strategy Deployed by the Venom of the Spider Cupiennius salei to Potentiate Toxicity on Insects 1, , 2, , 2 2 Benjamin Clémençon * y, Lucia Kuhn-Nentwig * y, Nicolas Langenegger , Lukas Kopp , Steve Peigneur 3 , Jan Tytgat 3,*, Wolfgang Nentwig 2 and Benjamin P. Lüscher 4 1 Department of Nephrology and Hypertension, Inselspital, Bern University Hospital, Freiburgstrasse 15, 3010 Bern, Switzerland 2 Institute of Ecology and Evolution, University of Bern, Baltzerstrasse 6, 3012 Bern, Switzerland; [email protected] (N.L.); [email protected] (L.K.); [email protected] (W.N.) 3 Toxicology and Pharmacology, University of Leuven (KU Leuven), Campus Gasthuisberg, O & N 2, Herestraat 49, P.O. Box 922, 3000 Leuven, Belgium; [email protected] 4 National Institutes of Health, Bethesda, MD 20892, USA; [email protected] * Correspondence: [email protected] (B.C.); [email protected] (L.K.-N.); [email protected] (J.T.) B.C. and L.K.-N. contributed equally to this work. y Received: 12 March 2020; Accepted: 10 April 2020; Published: 12 April 2020 Abstract: The venom of Cupiennius salei is composed of dozens of neurotoxins, with most of them supposed to act on ion channels. Some insecticidal monomeric neurotoxins contain an α-helical part besides their inhibitor cystine knot (ICK) motif (type 1). Other neurotoxins have, besides the ICK motif, an α-helical part of an open loop, resulting in a heterodimeric structure (type 2). Due to their low toxicity, it is difficult to understand the existence of type 2 peptides. -

CSTX-13, a Highly Synergistically Acting Two-Chain Neurotoxic Enhancer in the Venom of the Spider Cupiennius Salei (Ctenidae)
CSTX-13, a highly synergistically acting two-chain neurotoxic enhancer in the venom of the spider Cupiennius salei (Ctenidae) Benno Wullschleger*, Lucia Kuhn-Nentwig*†, Jan Tromp‡, Urs Ka¨ mpfer‡, Johann Schaller‡, Stefan Schu¨ rch‡, and Wolfgang Nentwig* *Zoological Institute, University of Bern, Baltzerstrasse 6, CH-3012 Bern, Switzerland; and ‡Department of Chemistry and Biochemistry, University of Bern, Freiestrasse 3, CH-3012 Bern, Switzerland Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved June 22, 2004 (received for review April 1, 2004) The survival of the spider Cupiennius salei depends on its hunting cysteine in their composition. Some peptides have already been success, which largely relies on its immediately paralyzing multi- characterized and named the cupiennin 1 family. These peptides component venom. Here, we report on the isolation and charac- exhibit strong bactericidal activities in the submicromolar terization of CSTX-13, a neurotoxic enhancer in the spider venom. range, but also cytolytic and insecticidal activities. In addition, De novo elucidation of the disulfide bridge pattern of CSTX-13 and cupiennin 1a shows a high synergistic effect with the main the neurotoxin CSTX-1 by tandem MS revealed an identical ar- neurotoxin CSTX-1, facilitating a rapid paralysis (12, 13). Pos- rangement. However, in contrast to CSTX-1, CSTX-13 is a two-chain itive insecticidal cooperativity between the cytolytically active peptide with two interchain and two intrachain disulfide bridges. oxyopinins and neurotoxins is also reported for the spider Furthermore, the insecticidal activity of CSTX-13 is synergistically Oxyopes kitabensis (14). ؉ increased in the presence of K ions as well as of the cytolytic The second group involves neurotoxically active peptides with peptide cupiennin 1a. -

This Is Normal Text
A COMPARATIVE ANALYSIS OF GENE EXPRESSION AMONG CASTES OF THE TERMITE RETICULITERMES FLAVIPES USING EXPRESSED SEQUENCE TAGS (ESTS) AND A MICROARRAY by MATTHEW MICHAEL STELLER B.S., UNIVERSITY OF MINNESOTA-DULUTH, 2005 A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Entomology College of Agriculture KANSAS STATE UNIVERSITY Manhattan, Kansas 2009 Approved by: Major Professor Srini Kambhampati Copyright MATTHEW MICHEAL STELLER 2009 Abstract Termites (Isoptera) are separated into morphologically and behaviorally specialized castes of sterile workers and soldiers, and the reproductive alates. Previous research on eusocial insects has indicated that caste differentiation has a genetic basis. Although much has been studied about the genetic basis of caste differentiation and behavior in the honey bee, Apis mellifera, termites remain comparatively understudied. Therefore, my objective was to compare the gene expression patterns of different castes of the termite Reticulitermes flavipes based on EST analyses and a microarray. Soldier, worker, and alate caste and two larval life stage cDNA libraries were constructed, and ~15,000 randomly chosen clones were sequenced to compile an EST database. Putative gene functions were assigned based on a BLASTX Swissprot search. Categorical expression patterns for each library were compared using the in silico methods of BLAST2GO and r-statistics. I chose 2,240 unique-ESTs based on their putative function and sequence quality, which I used to fabricate a Combimatrix microarray. I used the microarray to compare expression levels between workers and soldiers from Kansas and Florida populations. Seventy to ninety percent of the sequences from the ESTs of each caste and life stages had no significant similarity to those in existing databases. -

CSTX-13 and the Multi-Component Venom of the Spider Cupiennius Salei
CCSSTTXX--1133 aanndd tthhee mmuullttii--ccoommppoonneenntt vveennoomm ooff tthhee ssppiiddeerr CCuuppiieennnniiuuss ssaalleeii Inauguraldissertation der Philosophisch-naturwissenschaftlichen Fakultät der Universität Bern vorgelegt von Benno Wullschleger von Zofingen Leiter der Arbeit: Prof. Dr. Wolfgang Nentwig Zoologisches Institut der Universität Bern CSTX-13 and the multi-component venom of the spider Cupiennius salei Inauguraldissertation der Philosophisch-naturwissenschaftlichen Fakultät der Universität Bern vorgelegt von Benno Wullschleger von Zofingen Leiter der Arbeit: Prof. Dr. Wolfgang Nentwig Zoologisches Institut der Universität Bern Von der Philosophisch-naturwissenschaftlichen Fakultät angenommen. Bern, den 16.12.2004 Der Dekan: Prof. Dr. P. Messerli für Monika INHALTSVERZEICHNIS INHALTSVERZEICHNIS Einleitung 001 Publikationen 003 MANUSKRIPT I 004 Benno Wullschleger, Lucia Kuhn-Nentwig, Jan Tromp, Urs Kämpfer, Johann Schaller, Stefan Schürch & Wolfgang Nentwig (2004). CSTX-13, a highly synergistically acting two-chain neurotoxic enhancer in the venom of the spider Cupiennius salei (Ctenidae). Proc. Natl. Acad. Sci. USA 101, 11251–11256. MANUSKRIPT II 015 Benno Wullschleger, Wolfgang Nentwig & Lucia Kuhn-Nentwig. Spider venom: Enhancement of venom efficacy mediated by different synergistic strategies in Cupiennius salei. Submitted. MANUSKRIPT III 032 Benno Wullschleger, Wolfgang Nentwig & Lucia Kuhn-Nentwig. Different effects of neurotoxic and cytolytically acting peptides of Cupiennius salei venom to insects. Zusammenfassung 046 Literatur 048 Danksagung 051 Curriculum vitae 052 EINLEITUNG EINLEITUNG Spinnen gehören mit 38'663 rezenten Arten (PLATNICK, 2004) nach den Insekten zu der erfolgreichsten Tiergruppe. Die ebenfalls zu den Arachniden zählenden Skorpione gelten als die ältesten Landtiere, welche sich wahrscheinlich schon vor 400 Millionen Jahren entwickelt haben. Die ältesten fossilen Spinnen stammen aus dem Devon und dürften über 350 Millionen Jahre alt sein (WUNDERLICH, 1986). -

Review Antimicrobial and Cytolytic Peptides of Venomous Arthropods
CMLS, Cell. Mol. Life Sci. 60 (2003) 2651–2668 1420-682X/03/122651-18 DOI 10.1007/s00018-003-3106-8 CMLS Cellular and Molecular Life Sciences © Birkhäuser Verlag, Basel, 2003 Review Antimicrobial and cytolytic peptides of venomous arthropods L. Kuhn-Nentwig Zoological Institute, University of Bern, Baltzerstrasse 6, 3012 Bern (Switzerland), Fax: +41 31 631 48 88, e-mail: [email protected],URL: http://www.zoology.unibe.ch/ecol/ Received 17 March 2003; received after revision 11 June 2003; accepted 17 June 2003 Abstract. As a response to invading microorganisms, the interact together, resulting in extremely rapid immobi- innate immune system of arthropods has evolved a com- lization and/or killing of prey or aggressors. This review plex arrangement of constitutive and inducible antimicro- provides an overview of antimicrobial peptides identified bial peptides that immediately destroy a large variety of in the hemolymph of venomous arthropods, and espe- pathogens. At the same time, venomous arthropods have cially of cytolytic peptides in their venom. For these pep- developed an additional offensive system in their venom tides a dual role is proposed: acting as antimicrobials as glands to subdue their prey items. In this complex venom well as increasing the potency of the venom by influenc- system, several enzymes, low-molecular-mass com- ing excitable cells. pounds, neurotoxins, antimicrobial and cytolytic peptides Key words. Antimicrobial peptides; cytolytic peptides; venom; arthropods; synergism. Introduction membrane of bacteria and parasitic protozoans. The un- structured antimicrobial peptides are electrically at- As strategies in antagonistic relationships, prokaryotic tracted to negatively charged groups of the cell surface, and eukaryotic organisms have developed hundreds of where they adopt an a-helical conformation and accumu- different cytolytic peptides and proteins during their evo- late on the membrane.