Zootaxa, Araneae, Mygalomorphae, Antrodiaetidae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogeography of the Caribbean Cyrtognatha Spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J
www.nature.com/scientificreports OPEN Biogeography of the Caribbean Cyrtognatha spiders Klemen Čandek1,6,7, Ingi Agnarsson2,4, Greta J. Binford3 & Matjaž Kuntner 1,4,5,6 Island systems provide excellent arenas to test evolutionary hypotheses pertaining to gene fow and Received: 23 July 2018 diversifcation of dispersal-limited organisms. Here we focus on an orbweaver spider genus Cyrtognatha Accepted: 1 November 2018 (Tetragnathidae) from the Caribbean, with the aims to reconstruct its evolutionary history, examine Published: xx xx xxxx its biogeographic history in the archipelago, and to estimate the timing and route of Caribbean colonization. Specifcally, we test if Cyrtognatha biogeographic history is consistent with an ancient vicariant scenario (the GAARlandia landbridge hypothesis) or overwater dispersal. We reconstructed a species level phylogeny based on one mitochondrial (COI) and one nuclear (28S) marker. We then used this topology to constrain a time-calibrated mtDNA phylogeny, for subsequent biogeographical analyses in BioGeoBEARS of over 100 originally sampled Cyrtognatha individuals, using models with and without a founder event parameter. Our results suggest a radiation of Caribbean Cyrtognatha, containing 11 to 14 species that are exclusively single island endemics. Although biogeographic reconstructions cannot refute a vicariant origin of the Caribbean clade, possibly an artifact of sparse outgroup availability, they indicate timing of colonization that is much too recent for GAARlandia to have played a role. Instead, an overwater colonization to the Caribbean in mid-Miocene better explains the data. From Hispaniola, Cyrtognatha subsequently dispersed to, and diversifed on, the other islands of the Greater, and Lesser Antilles. Within the constraints of our island system and data, a model that omits the founder event parameter from biogeographic analysis is less suitable than the equivalent model with a founder event. -

Cryptic Species Delimitation in the Southern Appalachian Antrodiaetus
Cryptic species delimitation in the southern Appalachian Antrodiaetus unicolor (Araneae: Antrodiaetidae) species complex using a 3RAD approach Lacie Newton1, James Starrett1, Brent Hendrixson2, Shahan Derkarabetian3, and Jason Bond4 1University of California Davis 2Millsaps College 3Harvard University 4UC Davis May 5, 2020 Abstract Although species delimitation can be highly contentious, the development of reliable methods to accurately ascertain species boundaries is an imperative step in cataloguing and describing Earth's quickly disappearing biodiversity. Spider species delimi- tation remains largely based on morphological characters; however, many mygalomorph spider populations are morphologically indistinguishable from each other yet have considerable molecular divergence. The focus of our study, Antrodiaetus unicolor species complex which contains two sympatric species, exhibits this pattern of relative morphological stasis with considerable genetic divergence across its distribution. A past study using two molecular markers, COI and 28S, revealed that A. unicolor is paraphyletic with respect to A. microunicolor. To better investigate species boundaries in the complex, we implement the cohesion species concept and employ multiple lines of evidence for testing genetic exchangeability and ecological interchange- ability. Our integrative approach includes extensively sampling homologous loci across the genome using a RADseq approach (3RAD), assessing population structure across their geographic range using multiple genetic clustering analyses that include STRUCTURE, PCA, and a recently developed unsupervised machine learning approach (Variational Autoencoder). We eval- uate ecological similarity by using large-scale ecological data for niche-based distribution modeling. Based on our analyses, we conclude that this complex has at least one additional species as well as confirm species delimitations based on previous less comprehensive approaches. -

Opiliones, Cyphophthalmi, Pettalidae) from Sri Lanka with a Discussion on the Evolution of Eyes in Cyphophthalmi
2006. The Journal of Arachnology 34:331–341 A NEW PETTALUS SPECIES (OPILIONES, CYPHOPHTHALMI, PETTALIDAE) FROM SRI LANKA WITH A DISCUSSION ON THE EVOLUTION OF EYES IN CYPHOPHTHALMI Prashant Sharma and Gonzalo Giribet1: Department of Organismic & Evolutionary Biology and Museum of Comparative Zoology, Harvard University, 16 Divinity Avenue, Cambridge, Massachusetts 02138, USA ABSTRACT. A new species of Cyphophthalmi (Opiliones) belonging to the Sri Lankan genus Pettalus is described and illustrated. Characterization of male and female genitalia and SEM illustrations are in- cluded, representing the first such analysis for the genus. This constitutes the first species of Pettalus to be described since 1897, although information on other morphospecies recently collected in Sri Lanka indicates that the number of species on the island is much higher than previously thought. The presence of eyes in pettalids is illustrated for the first time and the implications of the presence of eyes outside of Stylocellidae are discussed. Keywords: Gondwana, Pettalus lampetides, Sri Lanka A dearth of collections and plentitude of during redescription. Study of the specimens mysteries have long been the hallmarks of the of P. brevicauda was not resumed until two cyphophthalmid fauna of Sri Lanka, arguably recent cladistic analyses of the cyphophthal- the most enigmatic among this suborder of mid genera (Giribet & Boyer 2002) [these Opiliones. Only two species—the first one specimens are referred to, erroneously, as P. originally assigned to the genus Cyphophthal- cimiciformis in this publication, following re- mus—have been formally recognized, both description by Hansen & Sørensen (1904)] over two centuries ago: Pettalus cimiciformis and specifically of the family Pettalidae (Gi- (O. -

Swiss Prospective Study on Spider Bites
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Bern Open Repository and Information System (BORIS) Original article | Published 4 September 2013, doi:10.4414/smw.2013.13877 Cite this as: Swiss Med Wkly. 2013;143:w13877 Swiss prospective study on spider bites Markus Gnädingera, Wolfgang Nentwigb, Joan Fuchsc, Alessandro Ceschic,d a Department of General Practice, University Hospital, Zurich, Switzerland b Institute of Ecology and Evolution, University of Bern, Switzerland c Swiss Toxicological Information Centre, Associated Institute of the University of Zurich, Switzerland d Department of Clinical Pharmacology and Toxicology, University Hospital Zurich, Switzerland Summary per year for acute spider bites, with a peak in the summer season with approximately 5–6 enquiries per month. This Knowledge of spider bites in Central Europe derives compares to about 90 annual enquiries for hymenopteran mainly from anecdotal case presentations; therefore we stings. aimed to collect cases systematically. From June 2011 to The few and only anecdotal publications about spider bites November 2012 we prospectively collected 17 cases of al- in Europe have been reviewed by Maretic & Lebez (1979) leged spider bites, and together with two spontaneous no- [2]. Since then only scattered information on spider bites tifications later on, our database totaled 19 cases. Among has appeared [3, 4] so this situation prompted us to collect them, eight cases could be verified. The causative species cases systematically for Switzerland. were: Cheiracanthium punctorium (3), Zoropsis spinimana (2), Amaurobius ferox, Tegenaria atrica and Malthonica Aim of the study ferruginea (1 each). Clinical presentation was generally mild, with the exception of Cheiracanthium punctorium, Main objective: To systematically document the clinical and patients recovered fully without sequelae. -

Spiders of the Hawaiian Islands: Catalog and Bibliography1
Pacific Insects 6 (4) : 665-687 December 30, 1964 SPIDERS OF THE HAWAIIAN ISLANDS: CATALOG AND BIBLIOGRAPHY1 By Theodore W. Suman BISHOP MUSEUM, HONOLULU, HAWAII Abstract: This paper contains a systematic list of species, and the literature references, of the spiders occurring in the Hawaiian Islands. The species total 149 of which 17 are record ed here for the first time. This paper lists the records and literature of the spiders in the Hawaiian Islands. The islands included are Kure, Midway, Laysan, French Frigate Shoal, Kauai, Oahu, Molokai, Lanai, Maui and Hawaii. The only major work dealing with the spiders in the Hawaiian Is. was published 60 years ago in " Fauna Hawaiiensis " by Simon (1900 & 1904). All of the endemic spiders known today, except Pseudanapis aloha Forster, are described in that work which also in cludes a listing of several introduced species. The spider collection available to Simon re presented only a small part of the entire Hawaiian fauna. In all probability, the endemic species are only partly known. Since the appearance of Simon's work, there have been many new records and lists of introduced spiders. The known Hawaiian spider fauna now totals 149 species and 4 subspecies belonging to 21 families and 66 genera. Of this total, 82 species (5596) are believed to be endemic and belong to 10 families and 27 genera including 7 endemic genera. The introduced spe cies total 65 (44^). Two unidentified species placed in indigenous genera comprise the remaining \%. Seventeen species are recorded here for the first time. In the catalog section of this paper, families, genera and species are listed alphabetical ly for convenience. -

Tarantulas in the Pacific Northwest1
WSU Puyallup REC PLS-108 Updated July 2003 Tarantulas in the Pacific Northwest1 Tarantulas (Fig. 1) in the Pacific Northwest? Well, maybe not like the hairy monsters of the tropics, but some very interesting "atypical" species do occur here. Our species belong to the family Antrodiaetidae. One of our most common spiders is the folding-door spider, Antrodiaetus pacificus (Simon). It is a fairly large species, females ranging from 11 to 13 millimeters in length, males slightly smaller. They are generally dark brown to almost black in color with the abdomen purplish brown. Males are characterized by their long legs, slim bodies, and three tergites (hardened plates) on the abdomen. Females (Fig. 2) are more robust with only one tergite. These spiders excavate burrows in the soil or in damp, rotten wood, digging with a row of spines on each chelicer, known as a ratellum. The six to ten inch deep vertical shafts are lined with silk. The webbing extends beyond ground level as a short collar of camouflaged silk. The turret’s two sides may be drawn in by the occupant, forming two "doors" which meet in the middle. At night, Antrodiaetus assumes a foraging posture with its pedipalps and first pair of legs just touching the rim of silk at the mouth of the tube. In this position, the folding door spider can readily detect an insect moving above ground. The spider will leap out of its burrow with lightning speed, seize its victim, and drop back down, like a terrorizing Jack-in-the-box. When finished with its meal, it will add the insect's dry, dismembered body to a silk-covered trash pile at the bottom of its burrow. -
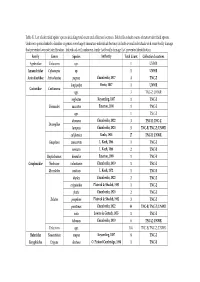
Table S1. List of Identified Spider Species Including Total Count and Collection Locations. Bold Cells Include Counts of Mature Identified Species
Table S1. List of identified spider species including total count and collection locations. Bold cells include counts of mature identified species. Unknown species linked to families or genera were largely immature individuals but may include several individuals with some bodily damage that prevented accurate identification. Individuals with unknown family had bodily damage that prevented identification. Family Genus Species Authority Total Count Collection Locations Agelenidae Unknown spp. 1 UNWR Amaurobiidae Cybaeopsis sp. 1 UNWR Antrodiaetidae Antrodiaetus pugnax Chamberlin, 1917 4 TNC-Z longipalpa Hentz, 1847 1 UNWR Corinnidae Castianeira spp. 3 TNC-Z; UNWR neglectus Keyserling, 1887 1 TNC-Z Drassodes saccatus Emerton, 1890 1 TNC-Z spp. 1 TNC-Z dromeus Chamberlin, 1922 3 TNC-B; TNC-Z Drassyllus lamprus Chamberlin, 1920 3 TNC-B; TNC-Z; UNWR californica Banks, 1904 17 TNC-B; UNWR Gnaphosa muscorum L. Koch, 1866 1 TNC-Z sericata L. Koch, 1866 2 TNC-B Haplodrassus hiemalis Emerton, 1909 1 TNC-B Gnaphosidae Nodocion voluntaries Chamberlin, 1919 1 TNC-Z Urozelotes rusticus L. Koch, 1872 1 TNC-B duplex Chamberlin, 1922 2 TNC-Z exiguioides Platnick & Shadab, 1983 1 TNC-Z fratis Chamberlin, 1920 2 TNC-Z Zelotes josephine Platnick & Shadab, 1983 3 TNC-Z puritanus Chamberlin, 1922 44 TNC-B; TNC-Z; UNWR sula Lowrie & Gertsch, 1955 1 TNC-Z tubuous Chamberlin, 1919 6 TNC-Z; UNWR Unknown spp. 184 TNC-B; TNC-Z; UNWR Hahniidae Neoantistea magna Keyserling, 1887 8 TNC-Z Linyphiidae Erigone dentosa O. Pickard-Cambridge, 1894 1 TNC-B spp. 1 TNC-Z Unknown spp. 13 TNC-Z; UNWR mccooki Montgomery, 1904 95 TNC-B; TNC-Z; UNWR Schizocosa minnesotensis Gertsch, 1934 25 TNC-B Lycosidae spp. -

The Effects of Native and Non-Native Grasses on Spiders, Their Prey, and Their Interactions
Spiders in California’s grassland mosaic: The effects of native and non-native grasses on spiders, their prey, and their interactions by Kirsten Elise Hill A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Science, Policy, and Management in the GRADUATE DIVISION of the University of California, Berkeley Committee in charge: Professor Joe R. McBride, Chair Professor Rosemary G. Gillespie Professor Mary E. Power Spring 2014 © 2014 Abstract Spiders in California’s grassland mosaic: The effects of native and non-native grasses on spiders, their prey, and their interactions by Kirsten Elise Hill Doctor of Philosophy in Environmental Science and Policy Management University of California, Berkeley Professor Joe R. McBride, Chair Found in nearly all terrestrial ecosystems, small in size and able to occupy a variety of hunting niches, spiders’ consumptive effects on other arthropods can have important impacts for ecosystems. This dissertation describes research into spider populations and their interactions with potential arthropod prey in California’s native and non-native grasslands. In meadows found in northern California, native and non-native grassland patches support different functional groups of arthropod predators, sap-feeders, pollinators, and scavengers and arthropod diversity is linked to native plant diversity. Wandering spiders’ ability to forage within the meadow’s interior is linked to the distance from the shaded woodland boundary. Native grasses offer a cooler conduit into the meadow interior than non-native annual grasses during midsummer heat. Juvenile spiders in particular, are more abundant in the more structurally complex native dominated areas of the grassland. -

Tarantulas and Social Spiders
Tarantulas and Social Spiders: A Tale of Sex and Silk by Jonathan Bull BSc (Hons) MSc ICL Thesis Presented to the Institute of Biology of The University of Nottingham in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy The University of Nottingham May 2012 DEDICATION To my parents… …because they both said to dedicate it to the other… I dedicate it to both ii ACKNOWLEDGEMENTS First and foremost I would like to thank my supervisor Dr Sara Goodacre for her guidance and support. I am also hugely endebted to Dr Keith Spriggs who became my mentor in the field of RNA and without whom my understanding of the field would have been but a fraction of what it is now. Particular thanks go to Professor John Brookfield, an expert in the field of biological statistics and data retrieval. Likewise with Dr Susan Liddell for her proteomics assistance, a truly remarkable individual on par with Professor Brookfield in being able to simplify even the most complex techniques and analyses. Finally, I would really like to thank Janet Beccaloni for her time and resources at the Natural History Museum, London, permitting me access to the collections therein; ten years on and still a delight. Finally, amongst the greats, Alexander ‘Sasha’ Kondrashov… a true inspiration. I would also like to express my gratitude to those who, although may not have directly contributed, should not be forgotten due to their continued assistance and considerate nature: Dr Chris Wade (five straight hours of help was not uncommon!), Sue Buxton (direct to my bench creepy crawlies), Sheila Keeble (ventures and cleans where others dare not), Alice Young (read/checked my thesis and overcame her arachnophobia!) and all those in the Centre for Biomolecular Sciences. -

Species Delimitation and Phylogeography of Aphonopelma Hentzi (Araneae, Mygalomorphae, Theraphosidae): Cryptic Diversity in North American Tarantulas
Species Delimitation and Phylogeography of Aphonopelma hentzi (Araneae, Mygalomorphae, Theraphosidae): Cryptic Diversity in North American Tarantulas Chris A. Hamilton1*, Daniel R. Formanowicz2, Jason E. Bond1 1 Auburn University Museum of Natural History and Department of Biological Sciences, Auburn University, Auburn, Alabama, United States of America, 2 Department of Biology, The University of Texas at Arlington, Arlington, Texas, United States of America Abstract Background: The primary objective of this study is to reconstruct the phylogeny of the hentzi species group and sister species in the North American tarantula genus, Aphonopelma, using a set of mitochondrial DNA markers that include the animal ‘‘barcoding gene’’. An mtDNA genealogy is used to consider questions regarding species boundary delimitation and to evaluate timing of divergence to infer historical biogeographic events that played a role in shaping the present-day diversity and distribution. We aimed to identify potential refugial locations, directionality of range expansion, and test whether A. hentzi post-glacial expansion fit a predicted time frame. Methods and Findings: A Bayesian phylogenetic approach was used to analyze a 2051 base pair (bp) mtDNA data matrix comprising aligned fragments of the gene regions CO1 (1165 bp) and ND1-16S (886 bp). Multiple species delimitation techniques (DNA tree-based methods, a ‘‘barcode gap’’ using percent of pairwise sequence divergence (uncorrected p- distances), and the GMYC method) consistently recognized a number of divergent and genealogically exclusive groups. Conclusions: The use of numerous species delimitation methods, in concert, provide an effective approach to dissecting species boundaries in this spider group; as well they seem to provide strong evidence for a number of nominal, previously undiscovered, and cryptic species. -

Phylogenomic Analysis and Revised Classification of Atypoid Mygalomorph Spiders (Araneae, Mygalomorphae), with Notes on Arachnid Ultraconserved Element Loci
Phylogenomic analysis and revised classification of atypoid mygalomorph spiders (Araneae, Mygalomorphae), with notes on arachnid ultraconserved element loci Marshal Hedin1, Shahan Derkarabetian1,2,3, Adan Alfaro1, Martín J. Ramírez4 and Jason E. Bond5 1 Department of Biology, San Diego State University, San Diego, CA, United States of America 2 Department of Biology, University of California, Riverside, Riverside, CA, United States of America 3 Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, MA, United States of America 4 Division of Arachnology, Museo Argentino de Ciencias Naturales ``Bernardino Rivadavia'', Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina 5 Department of Entomology and Nematology, University of California, Davis, CA, United States of America ABSTRACT The atypoid mygalomorphs include spiders from three described families that build a diverse array of entrance web constructs, including funnel-and-sheet webs, purse webs, trapdoors, turrets and silken collars. Molecular phylogenetic analyses have generally supported the monophyly of Atypoidea, but prior studies have not sampled all relevant taxa. Here we generated a dataset of ultraconserved element loci for all described atypoid genera, including taxa (Mecicobothrium and Hexurella) key to understanding familial monophyly, divergence times, and patterns of entrance web evolution. We show that the conserved regions of the arachnid UCE probe set target exons, such that it should be possible to combine UCE and transcriptome datasets in arachnids. We also show that different UCE probes sometimes target the same protein, and under the matching parameters used here show that UCE alignments sometimes include non- Submitted 1 February 2019 orthologs. Using multiple curated phylogenomic matrices we recover a monophyletic Accepted 28 March 2019 Published 3 May 2019 Atypoidea, and reveal that the family Mecicobothriidae comprises four separate and divergent lineages. -

Segmentation and Tagmosis in Chelicerata
Arthropod Structure & Development 46 (2017) 395e418 Contents lists available at ScienceDirect Arthropod Structure & Development journal homepage: www.elsevier.com/locate/asd Segmentation and tagmosis in Chelicerata * Jason A. Dunlop a, , James C. Lamsdell b a Museum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Invalidenstrasse 43, D-10115 Berlin, Germany b American Museum of Natural History, Division of Paleontology, Central Park West at 79th St, New York, NY 10024, USA article info abstract Article history: Patterns of segmentation and tagmosis are reviewed for Chelicerata. Depending on the outgroup, che- Received 4 April 2016 licerate origins are either among taxa with an anterior tagma of six somites, or taxa in which the ap- Accepted 18 May 2016 pendages of somite I became increasingly raptorial. All Chelicerata have appendage I as a chelate or Available online 21 June 2016 clasp-knife chelicera. The basic trend has obviously been to consolidate food-gathering and walking limbs as a prosoma and respiratory appendages on the opisthosoma. However, the boundary of the Keywords: prosoma is debatable in that some taxa have functionally incorporated somite VII and/or its appendages Arthropoda into the prosoma. Euchelicerata can be defined on having plate-like opisthosomal appendages, further Chelicerata fi Tagmosis modi ed within Arachnida. Total somite counts for Chelicerata range from a maximum of nineteen in Prosoma groups like Scorpiones and the extinct Eurypterida down to seven in modern Pycnogonida. Mites may Opisthosoma also show reduced somite counts, but reconstructing segmentation in these animals remains chal- lenging. Several innovations relating to tagmosis or the appendages borne on particular somites are summarised here as putative apomorphies of individual higher taxa.