Automated Brightness and Contrast Adjustment of Color Fundus Photographs for the Grading of Age-Related Macular Degeneration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
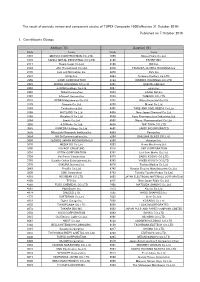
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

History of Topcon
1990 Entry into the GPS business. Released GPS 2008 Acquired US company VOXIS, Inc. and released 3D Released TOPCON RE SUPER, the world's receiver. laser scanner GLS-1000. 1963 first single-lens reflex camera with a TTL full- aperture metering system. History 1965 Released the retinal camera Type Ⅰ . Established Topcon Laser Systems, Inc. (currently Topcon 1994 Positioning Systems, Inc.) in California, USA. Acquired Advanced Grade Technology, entry into the machine control Released mobile survey system IP-S2, access to Established Tokyo Kogaku Seiki K.K. (currently Optonexus Co., Ltd.) in 2009 1969 business. accurate positional data, consecutive image of Fukushima Prefecture. surrounding areas and color 3D point cloud data of simply by installing in a vehicle and driving. Topcon Europe N.V. (currently Topcon Europe B.V.) Acquired JPS, Inc. in the USA. Started selling of precision 2000 GPS receivers and related system products. Topcon 1970 established in Rotterdam, The Netherlands in April. Topcon Instrument Corporation of America (currently Topcon Medical Systems, Inc.) in New York, USA (currently Established Topcon Medical Laser Systems, Inc. by acquiring located in New Jersey) established in October of the 2001 Established Topcon America Corporation in New Jersey, USA. 2010 retina and glaucoma business of OptiMedica (USA) and same year. As a holding company. Reorganized the subsidiaries in the USA and entered therapeutic laser market. Serve as the launching pad for further global expansion. divided into the positioning business and the eye care business. 2001 Used GPS to make dramatic improvements 201 1 Released Photocoagulator PASCAL Streamline. Original in high accuracy to world's first millimeter- multi-spot simultaneous laser pulse technology Released auto level AT-S3,AT-M3,AT-P3. -

International Smallcap Separate Account As of July 31, 2017
International SmallCap Separate Account As of July 31, 2017 SCHEDULE OF INVESTMENTS MARKET % OF SECURITY SHARES VALUE ASSETS AUSTRALIA INVESTA OFFICE FUND 2,473,742 $ 8,969,266 0.47% DOWNER EDI LTD 1,537,965 $ 7,812,219 0.41% ALUMINA LTD 4,980,762 $ 7,549,549 0.39% BLUESCOPE STEEL LTD 677,708 $ 7,124,620 0.37% SEVEN GROUP HOLDINGS LTD 681,258 $ 6,506,423 0.34% NORTHERN STAR RESOURCES LTD 995,867 $ 3,520,779 0.18% DOWNER EDI LTD 119,088 $ 604,917 0.03% TABCORP HOLDINGS LTD 162,980 $ 543,462 0.03% CENTAMIN EGYPT LTD 240,680 $ 527,481 0.03% ORORA LTD 234,345 $ 516,380 0.03% ANSELL LTD 28,800 $ 504,978 0.03% ILUKA RESOURCES LTD 67,000 $ 482,693 0.03% NIB HOLDINGS LTD 99,941 $ 458,176 0.02% JB HI-FI LTD 21,914 $ 454,940 0.02% SPARK INFRASTRUCTURE GROUP 214,049 $ 427,642 0.02% SIMS METAL MANAGEMENT LTD 33,123 $ 410,590 0.02% DULUXGROUP LTD 77,229 $ 406,376 0.02% PRIMARY HEALTH CARE LTD 148,843 $ 402,474 0.02% METCASH LTD 191,136 $ 399,917 0.02% IOOF HOLDINGS LTD 48,732 $ 390,666 0.02% OZ MINERALS LTD 57,242 $ 381,763 0.02% WORLEYPARSON LTD 39,819 $ 375,028 0.02% LINK ADMINISTRATION HOLDINGS 60,870 $ 374,480 0.02% CARSALES.COM AU LTD 37,481 $ 369,611 0.02% ADELAIDE BRIGHTON LTD 80,460 $ 361,322 0.02% IRESS LIMITED 33,454 $ 344,683 0.02% QUBE HOLDINGS LTD 152,619 $ 323,777 0.02% GRAINCORP LTD 45,577 $ 317,565 0.02% Not FDIC or NCUA Insured PQ 1041 May Lose Value, Not a Deposit, No Bank or Credit Union Guarantee 07-17 Not Insured by any Federal Government Agency Informational data only. -

July 12-14, 2017 Tokyo Big Sight
International M odern H ospital Show 2017 JULY 12-14, 2017 TOKYO BIG SIGHT JAPAN HOSPITAL ASSOCIATION NIPPON OMNI-MANAGEMENT ASSOCIATION East Hall3 East Hall East Hall1 Stage② Stage① Lunch Shop Rest Area Nursing Ideas Awards Rest Organizer’s Area Exhibition Supporting Organizations Information Lunch Shop Japan Hospital Association Exhibitor Presentations B 3 AMANO Company Limited E-6 Bunkyo City H-11 http://www.amano-grp.co.jp/ http://www.city.bunkyo.lg.jp 3DIT inc. A-47 http://3dit.jp Aqua Sougo Kikaku MUKASA Inc. D-6 BYTECH(DONGTAI) CO.,LTD. G-17 http://www.aqua-mukasa.com http://www.bytech-dt.com/? A Arai Medical Instruments, Inc. H-11 C Advanced Media.Inc. A-9 http://www.ami-inc.jp http://www.advanced-media.co.jp/ Array Corporation B-5 CAPITAL MEDICA CO.,Ltd. G-21 http://capimedi.com/ Aero Facility Co.,ltd. D-13 https://www.array.co.jp http://www.aero.co.jp/ AS ONE CORPORATION G-22 CardEx co.,ltd A-93 http://www.cardex.jp/ AERO SERVICE CORPORATION H-15 http://www.as-1.co.jp/ http://www.aeroserv.co.jp ASAHI ELECTRONICS LABOLATORY CO.,LTD A-26 CARECOM CO.,LTD. A-28 http://www.aelnet.co.jp https://www.carecom.jp/ AIHO corporation H-6 http://www.aiho.co.jp/ Assetment CORP. A-86 CAREFORCE. Inc G-2 https://www.assetment.net/ http://www.careforce.jp/ AINET SYSTEMS Inc. A-35 http://www.ains.co.jp/ Asterisk Inc. A-24 Carina System Co., Ltd. B-9 https://www.asx.co.jp http://www.carinasystem.co.jp/ AIPHONE CO.,LTD. -

Vista Imaging Approved DICOM Modality Interfaces November 30, 2019
VistA Imaging Approved DICOM Modality Interfaces November 30, 2019 VistA Imaging has successfully tested many modalities and interfaced them to VistA using DICOM. The following list of approved DICOM modalities was generated based on the most current information and will change as more information becomes available. A DICOM modality is considered “Supported” if it passes the VA’s internet validation test suite listed in the Joint VA/DoD DICOM Modality Conformance Requirements (Rev 3.0) document. Some modalities have superscript numbers which refer to the notes at the end of the document. If the software used on a modality is revised by the manufacturer, the modality will need to be re-validated. The VA retains the right to change modality testing requirements at any time as needed for VA medical care. These changes could require re-testing of the modalities. VA facilities are required to complete a quality review for the images produced by the device before it may be placed into clinical use. The results of the quality review must be filed with the National VistA Imaging Project Office. This list of approved DICOM modalities does not in any way constitute a product endorsement by the VA. It only states whether or not the listed product has passed the VA’s internet-based validation testing. Upgrades to the vendor devices can affect interoperability and may require re-validation. The validation process does not indicate how well the actual product will work operationally, either within the VA or elsewhere. Modality/Device Types Approved modalities and devices are grouped into the categories listed below. -

CDP Japan Water Security Report 2019
CDP Japan Water Security Report 2019 On behalf of 525 institutional investors with assets of USD 96 trillion CDP Japan Water Security Report 2019 | 2020 March Report writer Contents CDP Foreword 3 Report Writer Foreword 4 Water Security A List 2019 6 Scoring 7 Stories of Change 8 - Kao Corporation - Japan Tobacco Inc. Executive Summary 12 Response to CDP’s Water Security Questionnaire 14 Appendix 22 - CDP Water Security 2019 Japanese companies Please note that the names of companies in the text do not indicate their corporate status. Important Notice The contents of this report may be used by anyone providing acknowledgment is given to CDP. This does not represent a license to repackage or resell any of the data reported to CDP or the contributing authors and presented in this report. If you intend to repackage or resell any of the contents of this report, you need to obtain express permission from CDP before doing so. CDP has prepared the data and analysis in this report based on responses to the CDP 2019 information request. No representation or warranty (express or implied) is given by CDP as to the accuracy or completeness of the information and opinions contained in this report. You should not act upon the information contained in this publication without obtaining specific professional advice. To the extent permitted by law, CDP does not accept or assume any liability, responsibility or duty of care for any consequences of you or anyone else acting, or refraining to act, in reliance on the information contained in this report or for any decision based on it. -
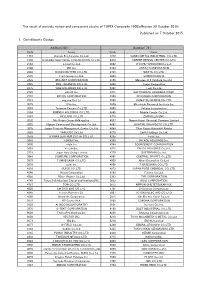
Published on 7 October 2015 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 30 October 2015) Published on 7 October 2015 1. Constituents Change Addition( 80 ) Deletion( 72 ) Code Issue Code Issue 1712 Daiseki Eco.Solution Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 1930 HOKURIKU ELECTRICAL CONSTRUCTION CO.,LTD. 2410 CAREER DESIGN CENTER CO.,LTD. 2183 Linical Co.,Ltd. 2692 ITOCHU-SHOKUHIN Co.,Ltd. 2198 IKK Inc. 2733 ARATA CORPORATION 2266 ROKKO BUTTER CO.,LTD. 2735 WATTS CO.,LTD. 2372 I'rom Group Co.,Ltd. 3004 SHINYEI KAISHA 2428 WELLNET CORPORATION 3159 Maruzen CHI Holdings Co.,Ltd. 2445 SRG TAKAMIYA CO.,LTD. 3204 Toabo Corporation 2475 WDB HOLDINGS CO.,LTD. 3361 Toell Co.,Ltd. 2729 JALUX Inc. 3371 SOFTCREATE HOLDINGS CORP. 2767 FIELDS CORPORATION 3396 FELISSIMO CORPORATION 2931 euglena Co.,Ltd. 3580 KOMATSU SEIREN CO.,LTD. 3079 DVx Inc. 3636 Mitsubishi Research Institute,Inc. 3093 Treasure Factory Co.,LTD. 3639 Voltage Incorporation 3194 KIRINDO HOLDINGS CO.,LTD. 3669 Mobile Create Co.,Ltd. 3197 SKYLARK CO.,LTD 3770 ZAPPALLAS,INC. 3232 Mie Kotsu Group Holdings,Inc. 4007 Nippon Kasei Chemical Company Limited 3252 Nippon Commercial Development Co.,Ltd. 4097 KOATSU GAS KOGYO CO.,LTD. 3276 Japan Property Management Center Co.,Ltd. 4098 Titan Kogyo Kabushiki Kaisha 3385 YAKUODO.Co.,Ltd. 4275 Carlit Holdings Co.,Ltd. 3553 KYOWA LEATHER CLOTH CO.,LTD. 4295 Faith, Inc. 3649 FINDEX Inc. 4326 INTAGE HOLDINGS Inc. 3660 istyle Inc. 4344 SOURCENEXT CORPORATION 3681 V-cube,Inc. 4671 FALCO HOLDINGS Co.,Ltd. 3751 Japan Asia Group Limited 4779 SOFTBRAIN Co.,Ltd. 3844 COMTURE CORPORATION 4801 CENTRAL SPORTS Co.,LTD. -

Survey of Integrated Reports in Japan 2018
Survey of Integrated Reports in Japan 2018 Integrated Reporting Center of Excellence KPMG in Japan March 2019 home.kpmg/jp Message from global thought leaders In this, the fifth year this survey report has been issued, KPMG has solicited the observations of thought leaders on corporate reporting. Japanese businesses pride themselves on a longer-term focus compared to the rest of the world, where prioritizing short-term gains has too often become the norm. Focusing on long-term value creation and taking into account all of the resources an organization uses is the sustainable, profitable, and proven way to manage a business. Many Japanese businesses are just beginning to implement integrated thinking and reporting. The findings in this report show that senior management must take ownership to spread integrated thinking in their businesses – not just in accounting but in strategy, operations, marketing, and the rest of the company as well. This report makes me optimistic and excited for Japanese business leaders as they strive to think, act, and communicate in an integrated and sustainable way. Dominic Barton ― International Integrated Reporting Council, Chairman Integrated Reporting improves communication between companies and investors and where most effective, sets the stage for enhanced corporate value creation over the mid to long-term. Investors desire integrated reports which provide comprehensive information disclosure useful for making investment decisions. The International Corporate Governance Network (ICGN) has encouraged integrated reporting for many years. In 2015, ICGN s Disclosure and Transparency Committee ’ released Guidance on Integrated Business Reporting. The Guidance amplifies that strategic decisions should be based on factors that are broader than those reflected in the financial statements. -

100498531.Pdf
\ Japan smarl community AⅡiance JSCA Japan smart communityAⅢance Pan Smart c.mmunlt 111ance 口0 ^ ^ 可借過N]喰愈明 As the world has become more and more concerned about climate change and the use of renewable energies such as solar povver and vvind po゛1er is expected to increase, the smart grid has been a廿racting worldwide attention as a means to achieve a stable renewable energy supply and realize an e仟ective and e仟icient electric grid system integrating information techn010gy. It is expected that large smart 引id markets wi11 emerge in Europe, Asia and the united states and that more comprehensive activities toward a smart community WⅢ develop.1n order for Japanese industry to participate in these activities, it is necessary to accelerate domestic smart grid・related activities, and a process that encourages public and private sector discussion of common goals, such as achieving global standardization and sustainable social system development,is also needed. This Process vvould also enable companies to vvork together to address issues that 、Nould otherwise be di仟icult for them to resolve by themselves. Considering this, the Ne、N Energy and lndustrial Techn010gy Development organization (NEDO) established the Japan smart community AⅡiance (JSCA) to strengthen c011aboration among a ゛1ide range of concerned organizations and also conduct activities of mutualinterest, such as dissemination of information and preparation of roadmaps to achieve global standardization. JSCA has members from the electric povver, gas, automobile,information and communications, electric machinery, construction and trading industries as we11 as the public sector and academia.(A Iist of members is included later in this brochure.) 1t is 、Norking to facilitate the development of a Smart community through the c011ective e什ort of the public and private sectors as an a11・Japan team by planning and implementing activities that benefit Japan's economic community. -

Patenting by Organizations Report, 1999
PATENTING BY ORGANIZATIONS 1999 April 2000 U.S. PATENT AND TRADEMARK OFFICE OFFICE FOR PATENT AND TRADEMARK INFORMATION / TAF PROGRAM PK3 - Suite 441, WASHINGTON, DC 20231 tel (703) 306-2600 / FAX (703) 306-2737 A TECHNOLOGY ASSESSMENT AND FORECAST REPORT Patenting By Organizations 1999 This report, prepared from the Technology Assessment and Forecast (TAF) database, profiles utility patents (i.e., patents for inventions) granted during calendar year 1999. Part A1 Part A1 presents patent counts by origin, U.S. and foreign. Individual counts are also presented for each of the top 36 patenting countries. Patent origin is determined by the residence of the first-named inventor listed on a patent. Patent ownership-category information reflects ownership at the time of patent grant and does not reflect subsequent changes in ownership. If more than one assignee (the entity, if any, to which the patent rights have been legally assigned) was declared at the time of grant, a patent is attributed to the ownership-category of the first-named assignee. The "U.S. Corporations" and "Foreign Corporations" ownership categories count predominantly corporate patents; however, patents assigned to other organizations such as small businesses, nonprofit organizations, universities, etc. are also included in these categories. While the "U.S. Government" ownership category includes only patents granted to the Federal Government, no such distinction is made for the "Foreign Government" ownership category. The "U.S. Individuals" and "Foreign Individuals" ownership categories include patents for which ownership was assigned to an individual as well as patents for which no assignment of ownership was made at the time of grant. -

Company Vendor ID (Decimal Format) (AVL) Ditest Fahrzeugdiagnose Gmbh 4621 @Pos.Com 3765 0XF8 Limited 10737 1MORE INC
Vendor ID Company (Decimal Format) (AVL) DiTEST Fahrzeugdiagnose GmbH 4621 @pos.com 3765 0XF8 Limited 10737 1MORE INC. 12048 360fly, Inc. 11161 3C TEK CORP. 9397 3D Imaging & Simulations Corp. (3DISC) 11190 3D Systems Corporation 10632 3DRUDDER 11770 3eYamaichi Electronics Co., Ltd. 8709 3M Cogent, Inc. 7717 3M Scott 8463 3T B.V. 11721 4iiii Innovations Inc. 10009 4Links Limited 10728 4MOD Technology 10244 64seconds, Inc. 12215 77 Elektronika Kft. 11175 89 North, Inc. 12070 Shenzhen 8Bitdo Tech Co., Ltd. 11720 90meter Solutions, Inc. 12086 A‐FOUR TECH CO., LTD. 2522 A‐One Co., Ltd. 10116 A‐Tec Subsystem, Inc. 2164 A‐VEKT K.K. 11459 A. Eberle GmbH & Co. KG 6910 a.tron3d GmbH 9965 A&T Corporation 11849 Aaronia AG 12146 abatec group AG 10371 ABB India Limited 11250 ABILITY ENTERPRISE CO., LTD. 5145 Abionic SA 12412 AbleNet Inc. 8262 Ableton AG 10626 ABOV Semiconductor Co., Ltd. 6697 Absolute USA 10972 AcBel Polytech Inc. 12335 Access Network Technology Limited 10568 ACCUCOMM, INC. 10219 Accumetrics Associates, Inc. 10392 Accusys, Inc. 5055 Ace Karaoke Corp. 8799 ACELLA 8758 Acer, Inc. 1282 Aces Electronics Co., Ltd. 7347 Aclima Inc. 10273 ACON, Advanced‐Connectek, Inc. 1314 Acoustic Arc Technology Holding Limited 12353 ACR Braendli & Voegeli AG 11152 Acromag Inc. 9855 Acroname Inc. 9471 Action Industries (M) SDN BHD 11715 Action Star Technology Co., Ltd. 2101 Actions Microelectronics Co., Ltd. 7649 Actions Semiconductor Co., Ltd. 4310 Active Mind Technology 10505 Qorvo, Inc 11744 Activision 5168 Acute Technology Inc. 10876 Adam Tech 5437 Adapt‐IP Company 10990 Adaptertek Technology Co., Ltd. 11329 ADATA Technology Co., Ltd. -

PV Celltech 2020 Online Provided a New Opportunity for the Global PV Sector to Participate at This Flagship Event by PV-Tech
400+ ATTENDEES REGISTERED 27 27-29 October 2020 PRESENTATIONS 9+ HOURS OF CONTENT Post ShowOnline 2020 Report 170+ COMPANIES ATTENDED PV CellTech 2020 Online provided a new opportunity for the global PV sector to participate at this flagship event by PV-Tech. By hosting the event online, key stakeholders in PV manufacturing and technology across almost every country and region worldwide were able to listen to 27 presentations from the companies driving the PV technology roadmap over the next few years. The event proved pivotal in framing the level of market adoption from n-type cell variants, in particular n-PERT/TOPCon and heterojunction architectures. It was clear that the migration from p-type mono PERC to these higher efficiency formats remained on the roadmaps of all leading PV producers today, with the phasing of the p-type to n-type transition being the one factor yet to be agreed on by the industry as a whole. In the meantime, efficiency gains using the mainstream p-mono PERC cell type continue to be made with 24% in volume production the new goal from the 100-GW-plus levels of capacity now installed globally. I would like to thank our sponsors and speakers who made the event such a success, and I look forward to our next event in April 2021. Finlay Colville Head of Research, PV Tech celltech.solarenergyevents.com | email: [email protected] Contact us to learn more about Thank you to our 2020 Partners! partnering GOLD SILVER SUPPORTING celltech.solarenergyevents.com | email: [email protected] Speakers Shubham