E5864.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibians in Zootaxa: 20 Years Documenting the Global Diversity of Frogs, Salamanders, and Caecilians
Zootaxa 4979 (1): 057–069 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Review ZOOTAXA Copyright © 2021 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4979.1.9 http://zoobank.org/urn:lsid:zoobank.org:pub:972DCE44-4345-42E8-A3BC-9B8FD7F61E88 Amphibians in Zootaxa: 20 years documenting the global diversity of frogs, salamanders, and caecilians MAURICIO RIVERA-CORREA1*+, DIEGO BALDO2*+, FLORENCIA VERA CANDIOTI3, VICTOR GOYANNES DILL ORRICO4, DAVID C. BLACKBURN5, SANTIAGO CASTROVIEJO-FISHER6, KIN ONN CHAN7, PRISCILLA GAMBALE8, DAVID J. GOWER9, EVAN S.H. QUAH10, JODI J. L. ROWLEY11, EVAN TWOMEY12 & MIGUEL VENCES13 1Grupo Herpetológico de Antioquia - GHA and Semillero de Investigación en Biodiversidad - BIO, Universidad de Antioquia, Antioquia, Colombia [email protected]; https://orcid.org/0000-0001-5033-5480 2Laboratorio de Genética Evolutiva, Instituto de Biología Subtropical (CONICET-UNaM), Facultad de Ciencias Exactas Químicas y Naturales, Universidad Nacional de Misiones, Posadas, Misiones, Argentina [email protected]; https://orcid.org/0000-0003-2382-0872 3Unidad Ejecutora Lillo, Consejo Nacional de Investigaciones Científicas y Técnicas - Fundación Miguel Lillo, 4000 San Miguel de Tucumán, Argentina [email protected]; http://orcid.org/0000-0002-6133-9951 4Laboratório de Herpetologia Tropical, Universidade Estadual de Santa Cruz, Departamento de Ciências Biológicas, Rodovia Jorge Amado Km 16 45662-900 Ilhéus, Bahia, Brasil [email protected]; https://orcid.org/0000-0002-4560-4006 5Florida Museum of Natural History, University of Florida, 1659 Museum Road, Gainesville, Florida, 32611, USA [email protected]; https://orcid.org/0000-0002-1810-9886 6Laboratório de Sistemática de Vertebrados, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Av. -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Redalyc.Reproductive Features of Chaltenobatrachus Grandisonae
Revista Chilena de Historia Natural ISSN: 0716-078X [email protected] Sociedad de Biología de Chile Chile CISTERNAS, JAVIERA; CORREA, CLAUDIO; VELÁSQUEZ, NELSON; PENNA, MARIO Reproductive features of Chaltenobatrachus grandisonae (Anura: Batrachylidae) within a protected area in Patagonia, Chile Revista Chilena de Historia Natural, vol. 86, núm. 3, 2013, pp. 365-368 Sociedad de Biología de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=369944186013 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative REPRODUCTION OF CHALTENOBATRACHUS GRANDISONAE 365 REVISTA CHILENA DE HISTORIA NATURAL Revista Chilena de Historia Natural 86: 365-368, 2013 © Sociedad de Biología de Chile NATURAL HISTORY NOTE Reproductive features of Chaltenobatrachus grandisonae (Anura: Batrachylidae) within a protected area in Patagonia, Chile Características reproductivas de Chaltenobatrachus grandisonae (Anura: Batrachylidae) en un área protegida en Patagonia, Chile JAVIERA CISTERNAS1,2,*, CLAUDIO CORREA1,3, NELSON VELÁSQUEZ2 & MARIO PENNA2 1Aumen o el Eco de los montes, Organización No Gubernamental, P. O. Box 393, Coyhaique, Chile 2Universidad de Chile, Facultad de Medicina, Instituto de Ciencias Biomédicas, P. O. Box 70005, Santiago, Chile 3Pontifi cia Universidad Católica de Chile, Departamento de Ecología, Alameda 340, P. O. Box 6513677, Santiago, Chile *Corresponding author: [email protected] Basso et al. (2011) assigned the monotypic Reproductive mode is defined by genus Chaltenobatrachus for the species a combination of characteristics including described originally as Telmatobius grandisonae breeding site, clutch structure, location of Lynch, 1975 (later transferred to the genus egg deposition, larval development site and Atelognathus by Lynch 1978). -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

Appendix B References
Final Tier 1 Environmental Impact Statement and Preliminary Section 4(f) Evaluation Appendix B, References July 2021 Federal Aid No. 999-M(161)S ADOT Project No. 999 SW 0 M5180 01P I-11 Corridor Final Tier 1 EIS Appendix B, References 1 This page intentionally left blank. July 2021 Project No. M5180 01P / Federal Aid No. 999-M(161)S I-11 Corridor Final Tier 1 EIS Appendix B, References 1 ADEQ. 2002. Groundwater Protection in Arizona: An Assessment of Groundwater Quality and 2 the Effectiveness of Groundwater Programs A.R.S. §49-249. Arizona Department of 3 Environmental Quality. 4 ADEQ. 2008. Ambient Groundwater Quality of the Pinal Active Management Area: A 2005-2006 5 Baseline Study. Open File Report 08-01. Arizona Department of Environmental Quality Water 6 Quality Division, Phoenix, Arizona. June 2008. 7 https://legacy.azdeq.gov/environ/water/assessment/download/pinal_ofr.pdf. 8 ADEQ. 2011. Arizona State Implementation Plan: Regional Haze Under Section 308 of the 9 Federal Regional Haze Rule. Air Quality Division, Arizona Department of Environmental Quality, 10 Phoenix, Arizona. January 2011. https://www.resolutionmineeis.us/documents/adeq-sip- 11 regional-haze-2011. 12 ADEQ. 2013a. Ambient Groundwater Quality of the Upper Hassayampa Basin: A 2003-2009 13 Baseline Study. Open File Report 13-03, Phoenix: Water Quality Division. 14 https://legacy.azdeq.gov/environ/water/assessment/download/upper_hassayampa.pdf. 15 ADEQ. 2013b. Arizona Pollutant Discharge Elimination System Fact Sheet: Construction 16 General Permit for Stormwater Discharges Associated with Construction Activity. Arizona 17 Department of Environmental Quality. June 3, 2013. 18 https://static.azdeq.gov/permits/azpdes/cgp_fact_sheet_2013.pdf. -

Species Assessment for Atlantic Coast Leopard Frog
Species Status Assessment Class: Amphibia Family: Ranidae Scientific Name: Lithobates [Rana] kauffeldi Common Name: Atlantic Coast leopard frog Species synopsis: More than a century of taxonomic confusion regarding the leopard frogs of the East Coast was resolved in 2012 with the publication of a genetic analysis (Newman et al. 2012) confirming that a third, cryptic species of leopard frog (Rana [= Lithobates] sp. nov.) occurs in southern New York, northern New Jersey, and western Connecticut. The molecular evidence strongly supported the distinction of this new species from the previously known northern (R. pipiens [= L. pipiens]) and southern (R. sphenocephala [=L. sphenocephalus]) leopard frogs. Rana kauffeldi is morphologically similar to R. sphenocephala and R. pipiens, but distinguishable by advertisement call, genetics, habitat, geographic distribution, and a combination of morphological characters (Feinberg et al. 2014). Bioacoustic evidence of the frog’s occurrence in southern New Jersey, Maryland, Delaware, and as far south as the Virginia/North Carolina border is available, thereby raising uncertainty about which species of leopard frog occur(s) presently and historically throughout the region. Some evidence suggests that Long Island might at one time have had two species: the southern leopard frog in the pine barrens and the Atlantic Coast leopard frog in coastal wetlands and the Hudson Valley. For simplicity’s sake, in this assessment we retain the name “Atlantic Coast leopard frog” even though much of the information available may also refer to the southern leopard frog or a combination of species (Feinberg et al. 2014). 1 I. Status a. Current and Legal Protected Status i. Federal ____ Not Listed______________________ Candidate? ___No____ ii. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
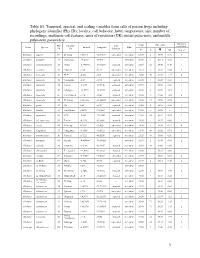
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff. -

Evaluating Methods for Phylogenomic Analyses, and a New Phylogeny for a Major Frog Clade
Molecular Phylogenetics and Evolution 119 (2018) 128–143 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evaluating methods for phylogenomic analyses, and a new phylogeny for a MARK major frog clade (Hyloidea) based on 2214 loci ⁎ Jeffrey W. Streichera,b, , Elizabeth C. Millera, Pablo C. Guerreroc,d, Claudio Corread, Juan C. Ortizd, Andrew J. Crawforde, Marcio R. Pief, John J. Wiensa a Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, USA b Department of Life Sciences, The Natural History Museum, London SW7 5BD, UK c Institute of Ecology and Biodiversity, Faculty of Sciences, University of Chile, 780-0024 Santiago, Chile d Facultad de Ciencias Naturales & Oceanográficas, Universidad de Concepción, Concepción, Chile e Department of Biological Sciences, Universidad de los Andes, A.A. 4976 Bogotá, Colombia f Departamento de Zoologia, Universidade Federal do Paraná, Curitiba, Paraná, Brazil ARTICLE INFO ABSTRACT Keywords: Phylogenomic approaches offer a wealth of data, but a bewildering diversity of methodological choices. These Amphibia choices can strongly affect the resulting topologies. Here, we explore two controversial approaches (binning Anura genes into “supergenes” and inclusion of only rapidly evolving sites), using new data from hyloid frogs. Hyloid Biogeography frogs encompass ∼53% of frog species, including true toads (Bufonidae), glassfrogs (Centrolenidae), poison Naive binning frogs (Dendrobatidae), and treefrogs (Hylidae). Many hyloid families are well-established, but relationships Phylogenomics among these families have remained difficult to resolve. We generated a dataset of ultraconserved elements Statistical binning (UCEs) for 50 ingroup species, including 18 of 19 hyloid families and up to 2214 loci spanning > 800,000 aligned base pairs. -

Resolving a Taxonomic and Nomenclatural Puzzle in Mantellid Frogs: Synonymization of Gephyromantis Azzurrae with G
ZooKeys 951: 133–157 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.951.51129 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research Resolving a taxonomic and nomenclatural puzzle in mantellid frogs: synonymization of Gephyromantis azzurrae with G. corvus, and description of Gephyromantis kintana sp. nov. from the Isalo Massif, western Madagascar Walter Cocca1, Franco Andreone2, Francesco Belluardo1, Gonçalo M. Rosa3,4, Jasmin E. Randrianirina5, Frank Glaw6, Angelica Crottini1,7 1 CIBIO, Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas, No 7, 4485-661 Vairão, Portugal 2 Sezione di Zoologia, Mu- seo Regionale di Scienze Naturali, Via G. Giolitti, 36, 10123 Torino, Italy 3 Institute of Zoology, Zoological Society of London, Regent’s Park, NW1 4RY London, UK 4 Centre for Ecology, Evolution and Environmental Changes (cE3c), Faculdade de Ciências da Universidade de Lisboa, Bloco C2, Campo Grande, 1749-016 Lisboa, Portugal 5 Parc Botanique et Zoologique de Tsimbazaza, BP 4096, Antananarivo 101, Madagascar 6 Zoologische Staatssammlung München (ZSM-SNSB), Münchhausenstraße 21, 81247 München, Germany 7 Departamento de Biologia, Faculdade de Ciências da Universidade do Porto, R. Campo Alegre, s/n, 4169- 007, Porto, Portugal Corresponding author: Angelica Crottini ([email protected]) Academic editor: A. Ohler | Received 14 February 2020 | Accepted 9 May 2020 | Published 22 July 2020 http://zoobank.org/5C3EE5E1-84D5-46FE-8E38-42EA3C04E942 Citation: Cocca W, Andreone F, Belluardo F, Rosa GM, Randrianirina JE, Glaw F, Crottini A (2020) Resolving a taxonomic and nomenclatural puzzle in mantellid frogs: synonymization of Gephyromantis azzurrae with G. -

OP-MOLB160269 Online 744..771
Evolutionary History of the Asian Horned Frogs (Megophryinae): Integrative Approaches to Timetree Dating in the Absence of a Fossil Record Stephen Mahony,*,1,2 Nicole M. Foley,1 S.D. Biju,2 and Emma C. Teeling*,1 1School of Biology and Environmental Science, University College Dublin, Belfield, Dublin, Ireland 2Systematics Lab, Department of Environmental Studies, University of Delhi, Delhi, India *Corresponding authors: E-mails: [email protected]; [email protected]. Associate editor: Beth Shapiro Abstract Downloaded from https://academic.oup.com/mbe/article-abstract/34/3/744/2919384 by guest on 06 August 2019 Molecular dating studies typically need fossils to calibrate the analyses. Unfortunately, the fossil record is extremely poor or presently nonexistent for many species groups, rendering such dating analysis difficult. One such group is the Asian horned frogs (Megophryinae). Sampling all generic nomina, we combined a novel 5 kb dataset composed of four nuclear and three mitochondrial gene fragments to produce a robust phylogeny, with an extensive external morpho- logical study to produce a working taxonomy for the group. Expanding the molecular dataset to include out-groups of fossil-represented ancestral anuran families, we compared the priorless RelTime dating method with the widely used prior-based Bayesian timetree method, MCMCtree, utilizing a novel combination of fossil priors for anuran phylogenetic dating. The phylogeny was then subjected to ancestral phylogeographic analyses, and dating estimates were compared with likely biogeographic vicariant events. Phylogenetic analyses demonstrated that previously proposed systematic hypotheses were incorrect due to the paraphyly of genera. Molecular phylogenetic, morphological, and timetree results support the recognition of Megophryinae as a single genus, Megophrys, with a subgenus level classification. -

Goliath Frogs Build Nests for Spawning – the Reason for Their Gigantism? Marvin Schäfera, Sedrick Junior Tsekanéb, F
JOURNAL OF NATURAL HISTORY 2019, VOL. 53, NOS. 21–22, 1263–1276 https://doi.org/10.1080/00222933.2019.1642528 Goliath frogs build nests for spawning – the reason for their gigantism? Marvin Schäfera, Sedrick Junior Tsekanéb, F. Arnaud M. Tchassemb, Sanja Drakulića,b,c, Marina Kamenib, Nono L. Gonwouob and Mark-Oliver Rödel a,b,c aMuseum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Berlin, Germany; bFaculty of Science, Laboratory of Zoology, University of Yaoundé I, Yaoundé, Cameroon; cFrogs & Friends, Berlin, Germany ABSTRACT ARTICLE HISTORY In contrast to its popularity, astonishingly few facts have become Received 16 April 2019 known about the biology of the Goliath Frog, Conraua goliath.We Accepted 7 July 2019 herein report the so far unknown construction of nests as spawning KEYWORDS sites by this species. On the Mpoula River, Littoral District, West Amphibia; Anura; Cameroon; Cameroon we identified 19 nests along a 400 m section. Nests Conraua goliath; Conrauidae; could be classified into three types. Type 1 constitutes rock pools parental care that were cleared by the frogs from detritus and leaf-litter; type 2 constitutes existing washouts at the riverbanks that were cleared from leaf-litter and/or expanded, and type 3 were depressions dug by the frogs into gravel riverbanks. The cleaning and digging activ- ities of the frogs included removal of small to larger items, ranging from sand and leaves to larger stones. In all nest types eggs and tadpoles of C. goliath were detected. All nest types were used for egg deposition several times, and could comprise up to three distinct cohorts of tadpoles.