Toxoplasmosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alaska Sea Lions and Seals
Alaska Sea Lions and Seals Blaire, Kate, Donovan, & Alex Biodiversity of Alaska 18 June 2017 https://www.stlzoo.org/files/3913/6260/5731/Sea-lion_RogerBrandt.jpg Similarities & Differences of Sea Lions and Seals Phocidae Family Otariidae Family cannot rotate back can rotate back flippers flippers; move like a marine under themselves to walk caterpillar on land mammals and run on land no external earflaps pinniped, “fin external earflaps footed” in use back flippers for Latin use front flippers for power when swimming power when swimming preyed upon by polar use front flippers for use back flippers for bears, orcas, steering when swimming steering when swimming and sharks food: krill, fish, lobster, food: squid, octopus, birds birds, and fish claws and fur on front no claws or hair on front flippers flippers Seals ("What’s the Difference “ 2017) Sea Lions Evolution • Both seals and sea lions are Pinnipeds • Descended from one ancestral line • Belong to order carnivora • Closest living relatives are bears and musteloids (diverged 50 million years ago) http://what-when-how.com/marine-mammals/pinniped-evolution- (Churchill 2015) marine-mammals/ http://www.chinadaily.com.cn/cndy/2009-04/24/content_7710231.htm Phylogenetics https://en.wikipedia.org/wiki/Pinniped Steller: Eumetopias jubatus http://www.arkive.org/stellers-sea-lion/eumetopias-jubatus/image-G62602.html Steller: Eumetopias jubatus • Classification (”Steller Sea Lion” 2017) Kingdom: Animalia Phylum: Chordata Class: Mamalia Order: Carnivora Family: Otarridae Genus: Eumetopias Species: -

Spotted Seals, Phoca Largha, in Alaska
Spotted Seals, Phoca largha, in Alaska Item Type article Authors Rugh, David J.; Shelden, Kim E. W.; Withrow, David E. Download date 09/10/2021 03:34:27 Link to Item http://hdl.handle.net/1834/26448 Spotted Seals, Phoca largha, in Alaska DAVID J. RUGH, KIM E. W. SHELDEN, and DAVID E. WITHROW Introduction mine the abundance, distribution, and lar), a 2-month difference in mating sea stock identification of marine mammals sons (effecting reproductive isolation), Under the reauthorization of the Ma that might have been impacted by com the whitish lanugo on newborn P largha rine Mammal Protection Act (MMPA) mercial fisheries in U.S. waters (Bra that is shed in utero in P vitulina, dif in 1988, and after a 5-year interim ex ham and DeMaster1). For spotted seals, ferences in the adult pelage of P largha emption period ending September 1995, Phoca largha, there were insufficient and P vitulina, and some differences in the incidental take of marine mammals data to determine incidental take lev cranial characteristics (Burns et aI., in commercial fisheries was authorized els. Accordingly, as a part of the MMAP, 1984). However, hybridization may if the affected populations were not ad the NMFS National Marine Mammal occur, based on evidence from morpho versely impacted. The Marine Mammal Laboratory (NMML) conducted a study logical intermediates and overlaps in Assessment Program (MMAP) of the of spotted seals in Alaska. The objec range (Bums et aI., 1984). As such, dif National Marine Fisheries Service tives of this study were to: I) provide a ferentiation of these two species in the (NMFS), NOAA, provided funding to review of literature pertaining to man field is very difficult. -

Mammal Species Native to the USA and Canada for Which the MIL Has an Image (296) 31 July 2021
Mammal species native to the USA and Canada for which the MIL has an image (296) 31 July 2021 ARTIODACTYLA (includes CETACEA) (38) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei - Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Eschrichtius robustus - Gray Whale 7. Megaptera novaeangliae - Humpback Whale BOVIDAE - cattle, sheep, goats, and antelopes 1. Bos bison - American Bison 2. Oreamnos americanus - Mountain Goat 3. Ovibos moschatus - Muskox 4. Ovis canadensis - Bighorn Sheep 5. Ovis dalli - Thinhorn Sheep CERVIDAE - deer 1. Alces alces - Moose 2. Cervus canadensis - Wapiti (Elk) 3. Odocoileus hemionus - Mule Deer 4. Odocoileus virginianus - White-tailed Deer 5. Rangifer tarandus -Caribou DELPHINIDAE - ocean dolphins 1. Delphinus delphis - Common Dolphin 2. Globicephala macrorhynchus - Short-finned Pilot Whale 3. Grampus griseus - Risso's Dolphin 4. Lagenorhynchus albirostris - White-beaked Dolphin 5. Lissodelphis borealis - Northern Right-whale Dolphin 6. Orcinus orca - Killer Whale 7. Peponocephala electra - Melon-headed Whale 8. Pseudorca crassidens - False Killer Whale 9. Sagmatias obliquidens - Pacific White-sided Dolphin 10. Stenella coeruleoalba - Striped Dolphin 11. Stenella frontalis – Atlantic Spotted Dolphin 12. Steno bredanensis - Rough-toothed Dolphin 13. Tursiops truncatus - Common Bottlenose Dolphin MONODONTIDAE - narwhals, belugas 1. Delphinapterus leucas - Beluga 2. Monodon monoceros - Narwhal PHOCOENIDAE - porpoises 1. Phocoena phocoena - Harbor Porpoise 2. Phocoenoides dalli - Dall’s Porpoise PHYSETERIDAE - sperm whales Physeter macrocephalus – Sperm Whale TAYASSUIDAE - peccaries Dicotyles tajacu - Collared Peccary CARNIVORA (48) CANIDAE - dogs 1. Canis latrans - Coyote 2. -

Historical Perspectives Nobuyuki Miyazaki (Born 4 August 1946)
Aquatic Mammals 2012, 38(2), 189-203, DOI 10.1578/AM.38.2.2012.189 Historical Perspectives Nobuyuki Miyazaki (born 4 August 1946) Nobuyuki Miyazaki began his career as a research associate at the University of Ryukyus, Japan, obtaining his Ph.D. in 1975 under Professor Nishiwaki. He established a Japanese research team focused on marine pollution and hazardous chemicals using marine mammals as an indica- tor species. Dr. Miyazaki organized the research project “Coastal Marine Environment” that was conducted by United Nations University, Ocean Research Institute of The University of Tokyo, and Iwate Prefecture. He worked as general coor- dinator of the Japanese Society for Promotion of Science’s Multilateral Core Univer sity Program “Coastal Marine Science” with other distinguished scientists from five Asian countries. In collabora- tion with Dr. Y. Naito, he developed an advanced Nobuyuki Miyazaki (Photo courtesy of John Anderson) data logger and camera logger, and he also estab- lished the “Bio-Logging Science” program at the University of Tokyo. Since 1990, he has conducted international ecological research of Lake Baikal in cooperation with colleagues from Russia, the United Kingdom, Belgium, Switzerland, and the United States. Dr. Miyazaki has published more than 270 English and 13 Japanese peer-reviewed papers, nine English and 51 Japanese books, and seven Eng lish and 109 Japanese reports. He also has given 316 presentations at national and inter- national conferences. 190 Miyazaki Seal Survey in Eurasian Waters in Collaboration with Russian Scientists Nobuyuki Miyazaki, Ph.D. Professor Emeritus, The University of Tokyo, Japan E-mail: [email protected] I. -
Summary of Protected Areas in Chad
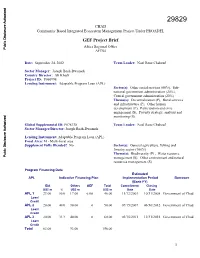
CHAD Community Based Integrated Ecosystem Management Project Under PROADEL GEF Project Brief Africa Regional Office Public Disclosure Authorized AFTS4 Date: September 24, 2002 Team Leader: Noel Rene Chabeuf Sector Manager: Joseph Baah-Dwomoh Country Director: Ali Khadr Project ID: P066998 Lending Instrument: Adaptable Program Loan (APL) Sector(s): Other social services (60%), Sub- national government administration (20%), Central government administration (20%) Theme(s): Decentralization (P), Rural services Public Disclosure Authorized and infrastructure (P), Other human development (P), Participation and civic engagement (S), Poverty strategy, analysis and monitoring (S) Global Supplemental ID: P078138 Team Leader: Noel Rene Chabeuf Sector Manager/Director: Joseph Baah-Dwomoh Lending Instrument: Adaptable Program Loan (APL) Focal Area: M - Multi-focal area Supplement Fully Blended? No Sector(s): General agriculture, fishing and forestry sector (100%) Theme(s): Biodiversity (P) , Water resource Public Disclosure Authorized management (S), Other environment and natural resources management (S) Program Financing Data Estimated APL Indicative Financing Plan Implementation Period Borrower (Bank FY) IDA Others GEF Total Commitment Closing US$ m % US$ m US$ m Date Date APL 1 23.00 50.0 17.00 6.00 46.00 11/12/2003 10/31/2008 Government of Chad Loan/ Credit APL 2 20.00 40.0 30.00 0 50.00 07/15/2007 06/30/2012 Government of Chad Loan/ Credit Public Disclosure Authorized APL 3 20.00 33.3 40.00 0 60.00 03/15/2011 12/31/2015 Government of Chad Loan/ Credit Total 63.00 93.00 156.00 1 [ ] Loan [X] Credit [X] Grant [ ] Guarantee [ ] Other: APL2 and APL3 IDA amounts are indicative. -

Status, Trends and Future Dynamics of Biodiversity and Ecosystems Underpinning Nature’S Contributions to People 1
CHAPTER 3 . STATUS, TRENDS AND FUTURE DYNAMICS OF BIODIVERSITY AND ECOSYSTEMS UNDERPINNING NATURE’S CONTRIBUTIONS TO PEOPLE 1 CHAPTER 2 CHAPTER 3 STATUS, TRENDS AND FUTURE DYNAMICS CHAPTER OF BIODIVERSITY AND 3 ECOSYSTEMS UNDERPINNING NATURE’S CONTRIBUTIONS CHAPTER TO PEOPLE 4 Coordinating Lead Authors Review Editors: Marie-Christine Cormier-Salem (France), Jonas Ngouhouo-Poufoun (Cameroon) Amy E. Dunham (United States of America), Christopher Gordon (Ghana) 3 CHAPTER This chapter should be cited as: Cormier-Salem, M-C., Dunham, A. E., Lead Authors Gordon, C., Belhabib, D., Bennas, N., Dyhia Belhabib (Canada), Nard Bennas Duminil, J., Egoh, B. N., Mohamed- (Morocco), Jérôme Duminil (France), Elahamer, A. E., Moise, B. F. E., Gillson, L., 5 Benis N. Egoh (Cameroon), Aisha Elfaki Haddane, B., Mensah, A., Mourad, A., Mohamed Elahamer (Sudan), Bakwo Fils Randrianasolo, H., Razaindratsima, O. H., Eric Moise (Cameroon), Lindsey Gillson Taleb, M. S., Shemdoe, R., Dowo, G., (United Kingdom), Brahim Haddane Amekugbe, M., Burgess, N., Foden, W., (Morocco), Adelina Mensah (Ghana), Ahmim Niskanen, L., Mentzel, C., Njabo, K. Y., CHAPTER Mourad (Algeria), Harison Randrianasolo Maoela, M. A., Marchant, R., Walters, M., (Madagascar), Onja H. Razaindratsima and Yao, A. C. Chapter 3: Status, trends (Madagascar), Mohammed Sghir Taleb and future dynamics of biodiversity (Morocco), Riziki Shemdoe (Tanzania) and ecosystems underpinning nature’s 6 contributions to people. In IPBES (2018): Fellow: The IPBES regional assessment report on biodiversity and ecosystem services for Gregory Dowo (Zimbabwe) Africa. Archer, E., Dziba, L., Mulongoy, K. J., Maoela, M. A., and Walters, M. (eds.). CHAPTER Contributing Authors: Secretariat of the Intergovernmental Millicent Amekugbe (Ghana), Neil Burgess Science-Policy Platform on Biodiversity (United Kingdom), Wendy Foden (South and Ecosystem Services, Bonn, Germany, Africa), Leo Niskanen (Finland), Christine pp. -

Periodic Status Review for the Steller Sea Lion
STATE OF WASHINGTON January 2015 Periodic Status Review for the Steller Sea Lion Gary J. Wiles The Washington Department of Fish and Wildlife maintains a list of endangered, threatened, and sensitive species (Washington Administrative Codes 232-12-014 and 232-12-011, Appendix E). In 1990, the Washington Wildlife Commission adopted listing procedures developed by a group of citizens, interest groups, and state and federal agencies (Washington Administrative Code 232-12-297, Appendix A). The procedures include how species listings will be initiated, criteria for listing and delisting, a requirement for public review, the development of recovery or management plans, and the periodic review of listed species. The Washington Department of Fish and Wildlife is directed to conduct reviews of each endangered, threatened, or sensitive wildlife species at least every five years after the date of its listing. The reviews are designed to include an update of the species status report to determine whether the status of the species warrants its current listing status or deserves reclassification. The agency notifies the general public and specific parties who have expressed their interest to the Department of the periodic status review at least one year prior to the five-year period so that they may submit new scientific data to be included in the review. The agency notifies the public of its recommendation at least 30 days prior to presenting the findings to the Fish and Wildlife Commission. In addition, if the agency determines that new information suggests that the classification of a species should be changed from its present state, the agency prepares documents to determine the environmental consequences of adopting the recommendations pursuant to requirements of the State Environmental Policy Act. -

UPDATE MARINE MAMMALS Circumpolar Marine Mammal Expert Group, CBMP-Marine
State of the Arctic Marine Biodiversity Report UPDATE MARINE MAMMALS Circumpolar Marine Mammal Expert Group, CBMP-Marine 2021 Polar bear with northern lights, Canada. Photo credit: Ondrej Prosicky/Shutterstock.com In 2017, the State of the Arctic Marine Biodiversity Report (SAMBR) synthesized data about biodiversity in Arctic marine ecosystems around the circumpolar Arctic. SAMBR highlighted observed changes The Circumpolar Biodiversity and relevant monitoring gaps. This document provides an update on Monitoring Program (CBMP) is the status of marine mammals in the circumpolar Arctic from 2015– an adaptive monitoring 2020 (scientific references for factual information and a more detailed program based on an version of the text herein can be found in Kovacs et al. 2021). international network of scientists, government Arctic endemic marine mammals are one focal group of the Circumpolar agencies, Indigenous Biodiversity Monitoring Program’s (CBMP) Arctic Marine Biodiversity organizations and conservation Monitoring Plan (CBMP-Marine Plan), along with sea ice biota, plankton, groups working together to benthos, marine fishes and seabirds. Networks of experts have harmonize and integrate efforts identified key elements, called Focal Ecosystem Components (FECs), to monitor the Arctic’s living within the Arctic marine ecosystem. Changes in the status of FECs resources. The CBMP organizes indicate changes in the broader marine environment. its efforts around the major This update was prepared by the Marine Mammals Expert Network, ecosystems of the Arctic: which works to coordinate monitoring and report scientific findings marine, freshwater, terrestrial regarding trends and their drivers in marine mammal populations and coastal. around the Arctic. In 2011, the CBMP Marine Expert Monitoring Group BACKGROUND published a circumpolar monitoring plan that describes Arctic endemic marine mammals live in close association with sea how Arctic states will compile, ice. -
Gazella Leptoceros
Gazella leptoceros Tassili N’Ajjer : Erg Tihodaïne. Algeria. © François Lecouat Pierre Devillers, Roseline C. Beudels-Jamar, , René-Marie Lafontaine and Jean Devillers-Terschuren Institut royal des Sciences naturelles de Belgique 71 Diagram of horns of Rhime (a) and Admi (b). Pease, 1896. The Antelopes of Eastern Algeria. Zoological Society. 72 Gazella leptoceros 1. TAXONOMY AND NOMENCLATURE 1.1. Taxonomy. Gazella leptoceros belongs to the tribe Antilopini, sub-family Antilopinae, family Bovidae, which comprises about twenty species in genera Gazella , Antilope , Procapra , Antidorcas , Litocranius , and Ammodorcas (O’Reagan, 1984; Corbet and Hill, 1986; Groves, 1988). Genus Gazella comprises one extinct species, and from 10 to 15 surviving species, usually divided into three sub-genera, Nanger , Gazella, and Trachelocele (Corbet, 1978; O’Reagan, 1984; Corbet and Hill, 1986; Groves, 1988). Gazella leptoceros is either included in the sub-genus Gazella (Groves, 1969; O’Reagan, 1984), or considered as forming, along with the Asian gazelle Gazella subgutturosa , the sub-genus Trachelocele (Groves, 1988). The Gazella leptoceros. Sidi Toui National Parks. Tunisia. species comprises two sub-species, Gazella leptoceros leptoceros of © Renata Molcanova the Western Desert of Lower Egypt and northeastern Libya, and Gazella leptoceros loderi of the western and middle Sahara. These two forms seem geographically isolated from each other and ecologically distinct, so that they must, from a conservation biology point of view, be treated separately. 1.2. Nomenclature. 1.2.1. Scientific name. Gazella leptoceros (Cuvier, 1842) Gazella leptoceros leptoceros (Cuvier, 1842) Gazella leptoceros loderi (Thomas, 1894) 1.2.2. Synonyms. Antilope leptoceros, Leptoceros abuharab, Leptoceros cuvieri, Gazella loderi, Gazella subgutturosa loderi, Gazella dorcas, var. -

SEALS and SEA LIONS in BRITISH COLUMBIA Sea Lion Comparison Five Species of Seals and Sea Lions (Pinnipeds) Are Found in British Columbia (B.C.) Waters
SEALS AND SEA LIONS IN BRITISH COLUMBIA Sea Lion Comparison Five species of seals and sea lions (pinnipeds) are found in British Columbia (B.C.) waters. Although commercial hunting of all marine mammals is prohibited in the Pacific Region, accidental Steller Sea Lions are commonly entanglement in fishing gear or debris, oil spills, conflicts with fisherman, and environmental mistaken for California Sea Lions contaminants all pose a threat to pinnipeds. which pass through B.C. in early winter and late spring. Look for Steller Sea Lions were listed as a species of Special Concern in Canada under the Species at Risk these key differences. Act (SARA) in 2005. People and boats can interfere with an animals’ ability to feed, communicate, rest, breed, and care for its young. Be cautious and quiet around pinnipeds, especially when passing haulouts, and avoid approaching closer than 100 meters. CALIFORNIA SEA LION STELLER SEA LION Only males in B.C. Males and females in B.C. Reporting Marine Mammal Incidents Broad snout Adults larger, Rescuing an injured or entangled seal or seal lion can be dangerous - do not attempt to touch or Prominent tan colour sagittal crest move an animal yourself. Observe from a distance and report any incidents of injured, entangled, Growling distressed, or dead marine mammals and sea turtles. If accidental contact occurs between a marine Long, narrow vocalizations snout mammal and your vessel or gear, regulations require you to immediately report it. Proper species identification (see back) is critical for documenting -

586 PROTECTION of ENDANGERED SPECIES of ANIMALS and PLANTS ORDINANCE Gazette Number Version Date Long Title LN 206 Of
Chapter: 586 PROTECTION OF ENDANGERED SPECIES OF Gazette Number Version Date ANIMALS AND PLANTS ORDINANCE Long title L.N. 206 of 2006 01/12/2006 An Ordinance to give effect in Hong Kong to the Convention on International Trade in Endangered Species of Wild Fauna and Flora signed in Washington D.C. on 3 March 1973; to regulate the import, introduction from the sea, export, re-export, and possession or control of certain endangered species of animals and plants and parts and derivatives of those species; and to provide for incidental and connected matters. [1 December 2006] L.N. 206 of 2006 (Originally 3 of 2006) Part: 1 PRELIMINARY L.N. 206 of 2006 01/12/2006 Section: 1 Short title L.N. 206 of 2006 01/12/2006 (1) This Ordinance may be cited as the Protection of Endangered Species of Animals and Plants Ordinance. (2) (Omitted as spent) Section: 2 Interpretation L.N. 130 of 2007 01/07/2007 Remarks: For the saving and transitional provisions relating to the amendments made by the Resolution of the Legislative Council (L.N. 130 of 2007), see paragraph (12) of that Resolution. (1) In this Ordinance, unless the context otherwise requires— "advertisement" (廣告), in relation to a specimen of a scheduled species, means any form of advertising that describes, makes reference or alludes in any other way to that scheduled species or specimen— (a) whether directly or indirectly; (b) whether orally, in writing in any language, diagrammatically, pictorially, by the use of symbols or photographs, or in any combination of them; and (c) whether or not the -

List of Common Names of Species Included in Appendices I and Ii
CMS Distr: General CONVENTION ON MIGRATORY UNEP/CMS/Inf.9.3 14 April 2008 SPECIES Original: English NINTH MEETING OF THE CONFERENCE OF THE PARTIES Rome, 1-5 December 2008 LIST OF COMMON NAMES OF SPECIES INCLUDED IN APPENDICES I AND II 1. The Secretariat compiled the attached document for its own purposes and, to the extent that it may also be useful to CMS Parties and others, it is reproduced here. The list is updated after each Meeting of the Conference of the Parties to incorporate the latest amendments to the Appendices, making use of inter alia the amendment proposals submitted by Parties. These often provide the common names of the proposed species in several of the four languages included in the attached list (English, French, Spanish and German). 2. The Secretariat would be grateful for any suggestions to complete the missing entries (insofar as common names exist for the species in question) and/or any necessary corrections to the existing entries. These may be sent to the Secretariat ( [email protected] ) prior to COP9 or left with the Secretariat during the conference or the preceding scientific council. For reasons of economy, documents are printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. List of Common Names, CMS Appendices I and II (with effect from May 2006) App. Taxon English French Spanish German I/II Accipitridae * eagles and hawks aigles, buses éperviers, milans, águilas, halcones y gavilanes