Honeybee Pollination and Apple Yields in Chitral, Pakistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Audit Report on the Accounts of District Government Chitral Audit Year 2016
AUDIT REPORT ON THE ACCOUNTS OF DISTRICT GOVERNMENT CHITRAL AUDIT YEAR 2016-17 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS AND ACRONYMS .............................................................. ii Preface ................................................................................................................... iv EXECUTIVE SUMMARY .................................................................................... v SUMMARY TABLES & CHARTS ................................................................... viii Table 1: Audit Work Statistics ................................................................................................. viii Table 2: Audit observations Classified by Categories (Rs in million) ...................................... viii Table 3: Outcome Statistics ........................................................................................................ ix Table 4: Table of Irregularities pointed out ................................................................................. x Table 5: Cost Benefit Ratio ......................................................................................................... x CHAPTER 1 ................................................................................................................................ 1 1.1 District Government Chitral ......................................................................................... 1 1.1.1 Introduction ................................................................................................................. -

An Indian Englishman
AN INDIAN ENGLISHMAN AN INDIAN ENGLISHMAN MEMOIRS OF JACK GIBSON IN INDIA 1937–1969 Edited by Brij Sharma Copyright © 2008 Jack Gibson All rights reserved. No part of this book may be reproduced, stored, or transmitted by any means—whether auditory, graphic, mechanical, or electronic—without written permission of both publisher and author, except in the case of brief excerpts used in critical articles and reviews. Unauthorized reproduction of any part of this work is illegal and is punishable by law. ISBN: 978-1-4357-3461-6 Book available at http://www.lulu.com/content/2872821 CONTENTS Preface vii Introduction 1 To The Doon School 5 Bandarpunch-Gangotri-Badrinath 17 Gulmarg to the Kumbh Mela 39 Kulu and Lahul 49 Kathiawar and the South 65 War in Europe 81 Swat-Chitral-Gilgit 93 Wartime in India 101 Joining the R.I.N.V.R. 113 Afloat and Ashore 121 Kitchener College 133 Back to the Doon School 143 Nineteen-Fortyseven 153 Trekking 163 From School to Services Academy 175 Early Days at Clement Town 187 My Last Year at the J.S.W. 205 Back Again to the Doon School 223 Attempt on ‘Black Peak’ 239 vi An Indian Englishman To Mayo College 251 A Headmaster’s Year 265 Growth of Mayo College 273 The Baspa Valley 289 A Half-Century 299 A Crowded Programme 309 Chini 325 East and West 339 The Year of the Dragon 357 I Buy a Farm-House 367 Uncertainties 377 My Last Year at Mayo College 385 Appendix 409 PREFACE ohn Travers Mends (Jack) Gibson was born on March 3, 1908 and J died on October 23, 1994. -
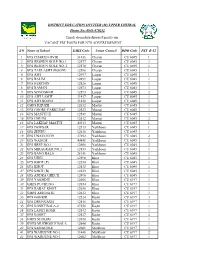
S.# Name of School EMIS Code Union Council DDO Code PST B-12 1
DISTRICT EDUCATION OFFICER (M) UPPER CHITRAL Phone No: 0943-470252 Email: [email protected] VACANT PST POSTS FOR NTS ADVERTISEMENT S.# Name of School EMIS Code Union Council DDO Code PST B-12 1 GPS CHARUN OVIR 31436 Charun CU 6045 1 2 GPS RESHUN GOLE NO.1 12577 Charun CU 6045 1 3 GPS RESHUN GOLE NO..2 12578 Charun CU 6045 1 4 GPS TAKLASHT (BOONI) 12596 Charun CU 6045 1 5 GPS AWI 12497 Laspur CU 6045 1 6 GPS BALIM 12499 Laspur CU 6045 1 7 GPS HERCHIN 12526 Laspur CU 6045 1 8 GPS RAMAN 12573 Laspur CU 6045 1 9 GPS SONOGHUR 12593 Laspur CU 6045 2 10 GPS AWI LASHT 31437 Laspur CU 6045 1 11 GPS AWI BOONI 31438 Laspur CU 6045 1 12 GMPS KHUZH 12632 Mastuj CU 6045 1 13 GPS GHORU PARKUSAP 12523 Mastuj CU 6045 1 14 GPS MASTUJ II 12549 Mastuj CU 6045 1 15 GPS CHUINJ 12512 Mastuj CU 6045 2 16 GPS LAKHAP MASTUJ 40313 Mastuj CU 6045 1 17 GPS DEWSAR 12513 Yarkhoon CU 6045 1 18 GPS ZHUPU 12610 Yarkhoon CU 6045 1 19 GPS UNAVOUCH 37292 Yarkhoon CU 6045 2 20 GPS WASUM 40841 Yarkhoon CU 6045 2 21 GPS BREP NO.1 12508 Yarkhoon CU 6045 2 22 GPS MIRAGRAM NO.2 12553 Yarkhoon CU 6045 1 23 GPS BANG BALA 28141 Yarkhoon CU 6045 1 24 GPS UJNU 12598 Khot CU 6045 1 25 GPS KHOT (P) 12534 Khot CU 6045 1 26 GPS KHOT 12532 Khot CU 6045 1 27 GPS KHOT (B) 12533 Khot CU 6045 1 28 GPS ANDRA GHECH 12496 Khot CU 6045 1 29 GPS YAKHDIZ 12606 Khot CU 6197 1 30 GMPS PUCHUNG 12654 Khot CU 6197 1 31 GPS RABAT KHOT 12656 Khot CU 6197 1 32 GMPS AMUNATE 12612 Khot CU 6197 1 33 GPS GOHKIR 12524 Kosht CU 6197 3 34 GPS DRUNGAGH 12516 Kosht CU 6197 1 35 GPS KOSHT BALA-2 27550 Kosht -

Language Documentation and Description
Language Documentation and Description ISSN 1740-6234 ___________________________________________ This article appears in: Language Documentation and Description, vol 17. Editor: Peter K. Austin Countering the challenges of globalization faced by endangered languages of North Pakistan ZUBAIR TORWALI Cite this article: Torwali, Zubair. 2020. Countering the challenges of globalization faced by endangered languages of North Pakistan. In Peter K. Austin (ed.) Language Documentation and Description 17, 44- 65. London: EL Publishing. Link to this article: http://www.elpublishing.org/PID/181 This electronic version first published: July 2020 __________________________________________________ This article is published under a Creative Commons License CC-BY-NC (Attribution-NonCommercial). The licence permits users to use, reproduce, disseminate or display the article provided that the author is attributed as the original creator and that the reuse is restricted to non-commercial purposes i.e. research or educational use. See http://creativecommons.org/licenses/by-nc/4.0/ ______________________________________________________ EL Publishing For more EL Publishing articles and services: Website: http://www.elpublishing.org Submissions: http://www.elpublishing.org/submissions Countering the challenges of globalization faced by endangered languages of North Pakistan Zubair Torwali Independent Researcher Summary Indigenous communities living in the mountainous terrain and valleys of the region of Gilgit-Baltistan and upper Khyber Pakhtunkhwa, northern -

Survey of Predatory Coccinellids (Coleoptera
Survey of Predatory Coccinellids (Coleoptera: Coccinellidae) in the Chitral District, Pakistan Author(s): Inamullah Khan, Sadrud Din, Said Khan Khalil and Muhammad Ather Rafi Source: Journal of Insect Science, 7(7):1-6. 2007. Published By: Entomological Society of America DOI: http://dx.doi.org/10.1673/031.007.0701 URL: http://www.bioone.org/doi/full/10.1673/031.007.0701 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Insect Science | www.insectscience.org ISSN: 1536-2442 Survey of predatory Coccinellids (Coleoptera: Coccinellidae) in the Chitral District, Pakistan Inamullah Khan, Sadrud Din, Said Khan Khalil and Muhammad Ather Rafi1 Department of Plant Protection, NWFP Agricultural University, Peshawar, Pakistan 1 National Agricultural Research Council, Islamabad, Pakistan Abstract An extensive survey of predatory Coccinellid beetles (Coleoptera: Coccinellidae) was conducted in the Chitral District, Pakistan, over a period of 7 months (April through October, 2001). -

Current Scenario and Threats to Ichthyo-Diversity in the Foothills of Hindu Kush: Addition to the Checklist of Coldwater Fishes of Pakistan
Pakistan J. Zool., vol. 48(1), pp. 285-288, 2016. Short Communication Current Scenario and Threats to Ichthyo-Diversity in the Foothills of Hindu Kush: Addition to the Checklist of Coldwater Fishes of Pakistan Arif Jan,* Abdul Rab, Rooh Ullah, Hussain Shah, Haroon, Iftikhar Ahmad, Muhammad Younas and Ikram Ullah Department of Zoology, Shaheed Benazir Bhutto University, Sheringal, Dir Upper. Article Information Received 16 January 2015 A B S T R A C T Revised 9 August 2015 Accepted 19 September 2015 Chitral, the pinnacle of Hindu Kush, draining 31 notable glaciers, is least studied for Ichthyo-faunal Available online 1 January 2016 diversity. This work explored the fish fauna and the risk factors for the Ichthyo-faunal diversity loss Authors’ Contributions at the foothills of Hindu Kush. A total of 21 fish species were collected from different parts and AJ has conducted the field work, tributaries of River Chitral, from Shandur up to Arandu, extending to Afghanistan border. Our analyzed the data and wrote the collection reported 4 fish species for the first time from Pakistan, namely Acanthocobitis article. HS, H and IA helped in the uropthalmus, Lepidopygnosis typus, Horalabiosa palaniensis, Horalabiosa joshuai. One species field work arrangements. MY, RU namely Nangra robusta is reported for the first time from River Chitral. Alluvial nature of rocks, and IU helped in literature search. construction of hydro projects and duck ponds, introduction of exotic species, erosion and AR helped in identification. sedimentation of rivers and streams, illegal fishing, and effluent discharges are the major concerns. Major threats to biodiversity loss need to be addressed for proper conservation of biodiversity as a Key words whole and Ichthyo-diversity in particular. -

Chitral Case Study April 26.Inddsec2:24 Sec2:24 4/27/2007 1:38:17 PM C H I T R a L
Chapter 3 Observing and Experiencing Flash Floods ocal knowledge on disaster preparedness in the Chitral the mountains. […] The fl ood blocked the river for 10 minutes District of Pakistan includes aspects related to people’s and it became a big lake and destroyed a water mill. The fl ood Lobservation and experience of fl ash fl oods, anticipation of continued until 9 pm. Some big stones are still stuck inside the fl oods, adjustment strategies, and communication strategies. valley.” (Narrated by Islamuddin, Aziz Urahman, Gul Muhammad Jan, Rashidullah, Khan Zarin and Ghulam Jafar, Gurin village, The people of Chitral have knowledge about the history and Gurin Gole, Shishi Koh valley, Lower Chitral) nature of fl ash fl oods in their own locality based on daily observation of their local surroundings, close ties to their “We were ready to take our goats up to the higher pastures environment for survival, and an accumulated understanding when eight days of discontinuous heavy rain fell from June 30th of their environment through generations. They have learned till July 9th 1978. We shifted our animals and family members to interpret their landscape and the physical indicators of past during that period to a nearby village. On July 7th, the river fl ash fl oods. They can also describe and explain how their own started to build up in the main course and to spill over. Some vulnerability to fl oods has changed over time. houses were destroyed. On July 9th even more houses were destroyed. The main fl ow came during the night of July 9th”. -

Dice Snakes in the Western Himalayas: Discussion of Potential Expansion Routes of Natrix Tessellata After Its Rediscovery in Pakistan
SALAMANDRA 49(4) 229–233 30 December 2013CorrespondenceISSN 0036–3375 Correspondence Dice Snakes in the western Himalayas: discussion of potential expansion routes of Natrix tessellata after its rediscovery in Pakistan Konrad Mebert 1 & Rafaqat Masroor 2 1) Section of Conservation Biology, Department of Environmental Sciences, University of Basel, St. Johanns-Vorstadt 10, CH-4056 Basel, Switzerland 2) Pakistan Museum of Natural History, Garden Avenue, Shakarparian, 44000-Islamabad, Pakistan Corresponding author: Konrad Mebert, e-mail: [email protected] Manuscript received: 3 September 2012 The dice snake (Natrix tessellata) has a large distribution stan Museum of Natural History and other experts in dif- range, extending from central (Germany) and southern Eu- ferent parts of northern Pakistan failed to find this species rope (Italy, Balkans) in the west, south to Egypt, and east as (e.g., Baig 2001, Khan 2002, Masroor 2012). far as northwestern China and Afghanistan (Mebert 2011a During recent herpetological surveys of wetlands con- and refs. therein). From Pakistan, N. tessellata has been doc- ducted in the framework of the Pakistan Wetlands Pro- umented only once (Wall 1911) with three specimens from gramme (PWP) by WWF-Pakistan, a single female an altitude of ca. 6,000 feet a.s.l. (~ 1,830 m) near Mastuj, of N. tessellata was collected at 1,845 m a.s.l. from the Tehsil of District Chitral, Khyber Pakhtunkhwa Province. Gahkuch Wetlands of Ghizer District, Gilgit-Baltistan, Pa- The only female, collected between 14 and 22 July 1910, laid kistan, on 20 August 2011. The dice snake was preserved as two eggs and subsequently died. -

DETAILS of Npos, SOCIAL WELFARE DEPARTMENT KHYBER PAKHTUNKHWA (Final Copy)
DETAILS OF NPOs, SOCIAL WELFARE DEPARTMENT KHYBER PAKHTUNKHWA (Final copy) (i) (ii) (iii) (iv) (v) (vi) (vii) (viii) (ix) (x) (xi) (xii) (xiii) (xiv) (xv) (xvi) (xvii) Name, Address & Contact No. Registration No. Sectors/ Target Size Latest Key Functionaries Persons in Effective Name & Value of Associate Bank Donor Means Mode Cross- Recruitme Detail of of NPO with Registering Function Area and Audited Control Moveable & d Entities Account Base of of Fund border nt Criminal Authority s Communit Accounts Immovable (if any) Details Paymen Payme Activiti Capabilitie /Administrati y available Assets (Bank, t nt es s ve Action (Yes /No) Branch & against NPO Account No.) (if any) 1 AAGHOSH WELFARE DSW/NWFP/254 Educatio Peshawar Mediu Yes Education Naseer Ahmad 01 Lack No;. Nil No. NA N.A N.A 07 Nil ORGANIZATION , ISLAMIA 9 n and m 03009399085 PUBLIC SCHOOL 09-03-2006 General aaghosh_2549@yahoo. BHATYAN CHARSADA Welfare com.com ROAD PESHAWAR 2 ABASEEN FOUNDATION DSW/NWFP/169 Educatio Peshawar mediu 2018 Education Dr. Mukhtiar Zaman 80 lac Nil --------- Both Bank Chequ Nil 20 Nil PAK, 3rd Floor, 272 Deans 9 n & m Tel: 0092 91 5603064 e Trade Centre, Peshawar 09.09.2000 health [email protected] Cantonment, Peshawar, . KPK, Pakistan. 3 Ahbab Welfare Organization, DSW/KPK/3490 Health Peshwar Small 2018 Dr. Habib Ullah 06 lac Nil ---------- Self Cash Cash Nil 08 Nil Sikandarpura G.t Rd 16.03.2011 educatio 0334-9099199 help Cheque Chequ n e 4 AIMS PAKISTAN DSW/NWFP/228 Patient’s KPK Mediu 2018 Patient’s Dr. Zia ul hasan 50 Lacs Nil 1721001193 Local Throug Bank Nil Nil 6-A B-3 OPP:Edhi home 9 Diabetic m Diabetic Welfare 0332 5892728, 690001 h Phase #05 Hayatabad 24,03.04 Welfare /Awareness 091-5892728 MIB Cheque Peshawar. -

Universal Service Fund (A Company Listed Under Section 42 of The
Registration Form Universal Service Fund (A company listed under Section 42 of the Companies Ordinance 1984) “Every Pakistani has the right to modern telecommunication services and we strive to provide that.” REGISTRATION FORM FOR USF PROJECT #__________________________ Name of Applicant: _________________________________ Registered Address: _________________________________ Postal Address: _________________________________ Phone: _________________________________ Fax: _________________________________ Email: _________________________________ Contact Person for correspondence: _________________________________ Person authorized for Registration: _________________________________ (attach copy of authorization) Nature of applicant’s License (copy attached): _________________________________ Telecom services authorized under the License: _________________________________ Date of Expiry of License: _________________________________ Licensed territory: _________________________________ Audited/Unaudited Report of USF Contributions as payable & paid todate: (summarize and attach copy of full report) _________________________________ _________________________________ _________________________________ Amount of any USF contributions outstanding to-date: ______________ Proof of payment: _________________________________ List of Consortium members, if registration is for consortium: _________________________________ _________________________________ _________________________________ Universal Service Fund, Habib Bank Tower, 5th Floor, Jinnah Avenue, -

Parcel Post Compendium Online Pakistan Post PKA PK
Parcel Post Compendium Online PK - Pakistan Pakistan Post PKA Basic Services CARDIT Carrier documents international Yes transport – origin post 1 Maximum weight limit admitted RESDIT Response to a CARDIT – destination Yes 1.1 Surface parcels (kg) 50 post 1.2 Air (or priority) parcels (kg) 50 6 Home delivery 2 Maximum size admitted 6.1 Initial delivery attempt at physical Yes delivery of parcels to addressee 2.1 Surface parcels 6.2 If initial delivery attempt unsuccessful, Yes 2.1.1 2m x 2m x 2m No card left for addressee (or 3m length & greatest circumference) 6.3 Addressee has option of paying taxes or Yes 2.1.2 1.5m x 1.5m x 1.5m Yes duties and taking physical delivery of the (or 3m length & greatest circumference) item 2.1.3 1.05m x 1.05m x 1.05m No 6.4 There are governmental or legally (or 2m length & greatest circumference) binding restrictions mean that there are certain limitations in implementing home 2.2 Air parcels delivery. 2.2.1 2m x 2m x 2m No 6.5 Nature of this governmental or legally (or 3m length & greatest circumference) binding restriction. 2.2.2 1.5m x 1.5m x 1.5m Yes (or 3m length & greatest circumference) 2.2.3 1.05m x 1.05m x 1.05m No 7 Signature of acceptance (or 2m length & greatest circumference) 7.1 When a parcel is delivered or handed over Supplementary services 7.1.1 a signature of acceptance is obtained Yes 3 Cumbersome parcels admitted No 7.1.2 captured data from an identity card are Yes registered 7.1.3 another form of evidence of receipt is No Parcels service features obtained 5 Electronic exchange of information -

Are the Kalasha Really of Greek Origin? the Legend of Alexander the Great and the Pre-Islamic World of the Hindu Kush1
Acta Orientalia 2011: 72, 47–92. Copyright © 2011 Printed in India – all rights reserved ACTA ORIENTALIA ISSN 0001-6483 Are the Kalasha really of Greek origin? The Legend of Alexander the Great and the Pre-Islamic World of the Hindu Kush1 Augusto S. Cacopardo Università di Firenze Abstract The paper refutes the claim that the Kalasha may be the descendants of the Greeks of Asia. First, traditions of Alexandrian descent in the Hindu Kush are examined on the basis of written sources and it is shown that such legends are not part of Kalasha traditional knowledge. Secondly, it is argued that the Kalasha were an integral part of the pre-Islamic cultural fabric of the Hindu Kush, and cannot be seen as intruders in the area, as legends of a Greek descent would want them. Finally, through comparative suggestions, it is proposed that possible similarities between the Kalasha and pre-Christian 1 Paper presented as a key-note address at the First International Conference on Language Documentation and Tradition, with a Special Interest in the Kalasha of the Hindu Kush Valleys, Himalayas – Thessaloniki, Greece, 7–9 November 2008. Scarcity of funds caused the scientific committee to decide to select for the forthcoming proceedings only linguistic papers. This is rather unfortunate because the inclusion in the volume of anthropological papers as well would have offered a good opportunity for comparing different views on the question of the Greek ascendancy of the Kalasha. 48 Augusto S. Cacopardo Europe are to be explained by the common Indo-European heritage rather than by more recent migrations and contacts.