Morphological Variation in the Human Tibia and Its Potential for Profile Estimation in Human Skeletal Remains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skier Tibia (Leg) Fractures
Skier Tibia (Leg) Fractures In years past, the prototypical ski fracture was sustained at the lower part of the outside of the leg in the region of the ankle. However, in the past 10 years, with the advent of the modern ski boots and improvements in binding, the most commonly seen lower leg skier fracture is the tibia (or shinbone) fracture. 10% of these fractures are associated with a collision. Thus, 90 % are associated with an isolated fall or noncontact type of injury, which is generally the result of binding malfunctions and inappropriate release. The most common mechanism leading to a tibia (leg) fracture is a forward fall. Risk factors for sustaining a skier tibia fracture include: beginners or novice skiers, less than 20 years of age, higher outdoor temperatures, and increased snow depth. Non-risk factors include ski lengths, icy conditions, and male versus female sex. The modern ski boot very closely resembles an extremely well padded short leg cast in the treatment of many orthopaedic lower extremity fractures. It of course goes to a much higher level than the former shorter boot top-level varieties. The binding release and designs have been based on the fracture strength of the adult tibia (shin) bone at the top of the modern ski boot. The treatment of most skier leg fractures includes a closed reduction and cast application for variable periods of time, with or without weight bearing allowed. However, severe misalignments of the bones can lead to later bony prominences that may be incompatible with snug, rigid, high fitting ski boots. -

Assessment, Management and Decision Making in the Treatment Of
Pediatric Ankle Fractures Anthony I. Riccio, MD Texas Scottish Rite Hospital for Children Update 07/2016 Pediatric Ankle Fractures The Ankle is the 2nd most Common Site of Physeal Injury in Children 10-25% of all Physeal Injuries Occur About the Ankle Pediatric Ankle Fractures Primary Concerns Are: • Anatomic Restoration of Articular Surface • Restoration of Symmetric Ankle Mortise • Preservation of Physeal Growth • Minimize Iatrogenic Physeal Injury • Avoid Fixation Across Physis in Younger Children Salter Harris Classification Prognosis and Treatment of Pediatric Ankle Fractures is Often Dictated by the Salter Harris Classification of Physeal Fractures Type I and II Fractures: Often Amenable to Closed Tx / Lower Risk of Physeal Arrest Type III and IV: More Likely to Require Operative Tx / Higher Risk of Physeal Arrest Herring JA, ed. Tachdjian’s Pediatric Orthopaedics, 5th Ed. 2014. Elsevier. Philadelphia, PA. ISOLATED DISTAL FIBULA FRACTURES Distal Fibula Fractures • The Physis is Weaker than the Lateral Ankle Ligaments – Children Often Fracture the Distal Fibula but…. – …ligamentous Injuries are Not Uncommon • Mechanism of Injury = Inversion of a Supinated Foot • SH I and II Fractures are Most Common – SH I Fractures: Average Age = 10 Years – SH II Fractures: Average Age = 12 Years Distal Fibula Fractures Lateral Ankle Tenderness SH I Distal Fibula Fracture vs. Lateral Ligamentous Injury (Sprain) Distal Fibula Fractures • Sankar et al (JPO 2008) – 37 Children – All with Open Physes, Lateral Ankle Tenderness + Normal Films – 18%: Periosteal -

Anatomy: Lower Leg, Knee, & Patella Positioning
Reading assignment: Lower Leg Anatomy: lower leg, knee, & Merrils, Vol. 1: Chapter 6 Film Critique #3 patella Lab demonstration Positioning: lower leg Positioning: knee Reading assignment: Knee Merrils, Vol. 1: Chapter 6 Film Critique #4 & Lab demonstration Positioning: intercondylar fossa Reading assignment: Intercondylar fossa and patella & patella Merrils, Vol. 1: Chapter 6 Lab demonstration Anatomy: Femur Reading assignment: Femur Positioning: Femur Merrils, Vol. 1: Chapters 6 & 7 Film Critique #5 Lab demonstration Leg…… The leg is composed of two long bones: Tibia – medial bone; second largest bone in the body Fibula – lateral bone The tibia has several anatomical features of note. See whether you can locate each on the diagram. Proximal end: Medial condyle Lateral condyle Tibial plateaus Intercondylar eminence Tibial tuberosity Body – features anterior crest Distal end: Medial malleolus Fibular notch The head of the fibula is located at its proximal end and has a pointed apex laterally. Distally, the fibular features the lateral malleolus. The articulations between the two leg bones are discussed on Screen 1.13. Knee….. The knee joint is the articulation between the femoral condyles and the tibial plateaus. Numerous soft tissues support and reinforce the knee, including the: Menisci Cruciate ligaments Collateral ligaments These supporting soft tissue structures are enclosed in a common joint capsule. The knee joint is of the hinge type, capable of flexion and extension only. The anterior knee joint is protected by the patella and patellofemoral joint. The patella is the largest and most constant sesamoid bone. It develops in the quadriceps femoris tendon between the ages of 3 and 5 years. -
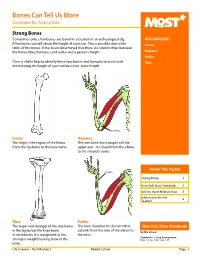
Bones Can Tell Us More Compiled By: Nancy Volk
Bones Can Tell Us More Compiled By: Nancy Volk Strong Bones Sometimes only a few bones are found in a location in an archeological dig. VOCABULARY A few bones can tell about the height of a person. This is possible due to the Femur ratios of the bones. It has been determined that there are relationships between the femur, tibia, humerus, and radius and a person’s height. Humerus Radius Here is a little help to identify these four bones and formulas to assist with Tibia determining the height of a person based on bone length. Humerus Femur: Humerus: The thigh is the region of the femur. The arm bone most people call the From the hip bone to the knee bone. upper arm. It is found from the elbow to the shoulder joints. Inside This Packet Radius Strong Bones 1 New York State Standards 1 Activity: Bone Relationships 2 Information for the Teacher 4 Tibia: Radius: The larger and stronger of the two bones The bone found in the forearm that New York State Standards in the leg below the knee bone. extends from the side of the elbow to Middle School In vertebrates It is recognized as the the wrist. Standard 4: Living Environment strongest weight bearing bone in the Idea 1: 1.2a, 1.2b, 1.2e, 1.2f body. Life Sciences - Post Module 3 Middle School Page 1 Activity: Bone Relationships MATERIALS NEEDED Skeleton Formulas: Tape Measure Bone relationship is represented by the following formulas: Directions and formulas P represents the person’s height. The last letter of each formula stands for the Calculator known length of the bone (femur, tibia, humerus, or radius) through measurement. -

Normative Values for Femoral Length, Tibial Length, Andthe Femorotibial
Article Normative Values for Femoral Length, Tibial Length, and the Femorotibial Ratio in Adults Using Standing Full-Length Radiography Stuart A Aitken MaineGeneral Medical Center, 35 Medical Center Parkway, Augusta, ME 04330, USA; [email protected] Abstract: Knowledge of the normal length and skeletal proportions of the lower limb is required as part of the evaluation of limb length discrepancy. When measuring limb length, modern standing full-length digital radiographs confer a level of clinical accuracy interchangeable with that of CT imaging. This study reports a set of normative values for lower limb length using the standing full-length radiographs of 753 patients (61% male). Lower limb length, femoral length, tibial length, and the femorotibial ratio were measured in 1077 limbs. The reliability of the measurement method was tested using the intra-class correlation (ICC) of agreement between three observers. The mean length of 1077 lower limbs was 89.0 cm (range 70.2 to 103.9 cm). Mean femoral length was 50.0 cm (39.3 to 58.4 cm) and tibial length was 39.0 cm (30.8 to 46.5 cm). The median side-to-side difference was 0.4 cm (0.2 to 0.7, max 1.8 cm) between 324 paired limbs. The mean ratio of femoral length to tibial length for the study population was 1.28:1 (range 1.16 to 1.39). A moderately strong inverse linear relationship (r = −0.35, p < 0.001, Pearson’s) was identified between tibial length and the Citation: Aitken, S.A. Normative corresponding femorotibial ratio. -

Tibial De-Rotational Osteotomies for Tibial Torsion
Tibial De-Rotational Osteotomies for Tibial Torsion Why does my child need tibial torsion surgery? What happens during the surgery? A lot of young children walk with their toes pointing in or First, the surgeon cracks the tibia and the smaller fibula bone out instead of straight ahead. The most common reason is next to it, usually just above the ankle. Surgically cracking a tibial torsion, a twist in the tibia bone of the lower leg. This bone is also known as an osteotomy. It is similar to breaking twist brings the knee and ankle out of alignment. The feet a bone, except that it is done on purpose. The surgeon respond by turning in (internal tibia torsion) or out (external weakens the tibia bone first by drilling holes through a small tibia torsion). Most of the time, tibial torsion gets better as a surgical opening. The next step is to rotate, or turn, the bone child exercises the leg muscles by walking and running. into correct alignment. The surgeon then places a pin in the bone just below the knee. The pin will be removed once the But when a child has spasticity, a condition in which muscle bone heals. In the meantime, your child will wear a cast that tone is very strong, tibial torsion can get worse instead starts at the pin and covers the leg and foot. The cast keeps of better as the child grows. Surgery helps children with the leg from moving while new bone grows. spasticity who can stand, but cannot walk or run normally because of tibial torsion. -

NCB® Proximal Tibia System Surgical Technique
NCB® Proximal Tibia System Surgical Technique NCB® Proximal Tibia System– Surgical Technique 3 Surgical Technique Table of Contents NCB Locking Plate Introduction 4 System for Proximal Tibia Plate Design 5 Screw Selection 5 Cable Fixation Options 6 MIS Radiolucent Targeting Device 7 System Features 7 Indications/Contraindications 8 Fracture Classification 8 Sample Cases 9 Preoperative Planning and Patient Positioning 11 Open Technique 12 Incision 12 Fracture Reduction 12 Optional: Bone Spacers 12 Insertion of NCB PT Plate 13 Insertion of NCB Screws 13 MIS Technique* 18 Plate Hole Numbering System 18 Incision and Fracture Reduction 18 Targeting Device Assembly 19 Insertion and Preliminary Fixation of NCB PT Plate 19 Insertion of NCB Screws in the Proximal Area 21 Insertion of NCB Screws in the Shaft 22 Implant Removal 24 Ordering Information 25 Implants 25 Graphic Case 28 Standard Instruments 29 MIS Instruments 30 Cannulated Option (Screws and Instruments) 32 * MIS Minimally Invasive Solutions™ Technique by Zimmer Planning Aid 33 4 NCB® Proximal Tibia System – Surgical Technique Introduction The NCB PT (Non-Contact Bridging for the Proximal Tibia) is an optimal plate solution for the treatment of complex fractures of the proximal tibia. The system allows for polyaxial screw placement (30°) with subsequent screw locking. Before locking, the screws can act as lag screws and be used for fracture reduction; a benefit which is not offered with standard locking systems. Implants are available with 2 or 3 proximal holes, left and right. Plate length In the locked mode, NCB PT Plate late varies from 5 to 9 shaft acts as an internal fixator without holes for the 2-proximal hole contact between the plate and the plate and between 3 and 13 shaft holes for the 3-proxi- bone surface reducing the risk of mal hole plate. -

Anatomy, Bony Pelvis and Lower Limb, Leg Bones
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Anatomy, Bony Pelvis and Lower Limb, Leg Bones Authors Austin J. Cantrell1; Matthew Varacallo2. Affiliations 1 University of Oklahoma College of Med. 2 Department of Orthopaedic Surgery, University of Kentucky School of Medicine Last Update: January 17, 2019. Introduction The leg is the region of the lower limb between the knee and the foot. It comprises two bones: the tibia and the fibula. The role of these two bones is to provide stability and support to the rest of the body, and through articulations with the femur and foot/ankle and the muscles attached to these bones, provide mobility and the ability to ambulate in an upright position. The tibia articulates with the femur at the knee joint. The knee joint consists of three compartments [1][2] medial tibiofemoral compartment lateral tibiofemoral compartment patellofemoral compartment At the ankle, the tibia and fibula create the articular surface for the talus. The ankle mortise is a specialized articulation providing support and optimizing motion and function through the ankle joint. A normal ankle joint ultimately optimizes and allows for physiologic mobility of the foot and its associated joints and articulations. The bones and fascia also divide the lower leg into four compartments [3][4] anterior compartment lateral compartment posterior compartment, superficial posterior compartment, deep Structure and Function The tibia is the second largest bone in the body and provides support for a significant portion of the weight-bearing forces transmitted from the rest of the body. -

Chapter 10 the Knee Joint
The Knee Joint • Knee joint – largest joint in body Chapter 10 – very complex The Knee Joint – primarily a hinge joint Manual of Structural Kinesiology Modified for Prentice WE: Arnheim’s principles of athletic training , ed 12, New R.T. Floyd, EdD, ATC, CSCS York, 2006, McGraw-Hill; from Saladin, KS: Anatomy &physiology: the unity of forms and function , ed 2, New York, 2001, McGraw- Hill. © 2007 McGraw-Hill Higher Education. All rights reserved. 10-1 © 2007 McGraw-Hill Higher Education. All rights reserved. 10-2 Bones Bones • Enlarged femoral condyles articulate on • Fibula - lateral enlarged tibial condyles – serves as the attachment for • Medial & lateral tibial condyles (medial & knee joint lateral tibial plateaus) - receptacles for structures femoral condyles – does not articulate • Tibia – medial with femur or patella – bears most of weight – not part of knee joint Modified from Anthony CP, Kolthoff NJ: Textbook of anatomy and physiology , ed 9, St. Louis, 1975, Mosby. © 2007 McGraw-Hill Higher Education. All rights reserved. 10-3 © 2007 McGraw-Hill Higher Education. All rights reserved. 10-4 Bones Bones • Patella • Key bony landmarks – sesamoid (floating) bone – Superior & inferior patellar poles – imbedded in quadriceps – Tibial tuberosity & patellar tendon – Gerdy’s tubercle – serves similar to a pulley – Medial & lateral femoral in improving angle of condyles pull, resulting in greater – Upper anterior medial tibial mechanical advantage in surface – Head of fibula knee extension Modified from Anthony CP, Kolthoff NJ: Textbook of anatomy and physiology , ed 9, St. Louis, 1975, Mosby. © 2007 McGraw-Hill Higher Education. All rights reserved. 10-5 © 2007 McGraw-Hill Higher Education. All rights reserved. -
Bones of the Lower Limb Doctors Notes Notes/Extra Explanation Editing File Objectives
Color Code Important Bones of the Lower Limb Doctors Notes Notes/Extra explanation Editing File Objectives Classify the bones of the three regions of the lower limb (thigh, leg and foot). Memorize the main features of the – Bones of the thigh (femur & patella) – Bones of the leg (tibia & Fibula) – Bones of the foot (tarsals, metatarsals and phalanges) Recognize the side of the bone. ﻻ تنصدمون من عدد ال رشائح نصها رشح زائد وملخصات واسئلة Some pictures in the original slides have been replaced with other pictures which are more clear BUT they have the same information and labels. Terminology (Team 434) شيء مرتفع /Eminence a small projection or bump Terminology (Team 434) Bones of thigh (Femur and Patella) Femur o Articulates (joins): (1) above with Acetabulum of hip bone to form the hip joint, (2) below with tibia and patella to form the knee joint. Body of femur (shaft) o Femur consists of: I. Upper end. II. Shaft. III. Lower end. Note: All long bones consist of three things: 1- upper/proximal end posterior 2- shaft anterior 3- lower/distal end I. Upper End of Femur The upper end contains: A. Head B. Neck C. Greater trochanter & D. Lesser trochanter A. Head: o Articulates (joins) with acetabulum of hip bone to form the hip joint. o Has a depression in the center called Fovea Capitis. o The fovea capitis is for the attachment of ligament of the head of Femur. o An artery called Obturator Artery passes along this ligament to supply head of Femur. B. Neck: o Connects head to the shaft. -

Bones of Lower Limb
BONES OF LOWER LIMB ANATOMY DEPARTMENT Dr. Sanaa Alshaarawi OBJECTIVES • At the end of the lecture the students should be able to: • Classify the bones of the three regions of the lower limb (thigh, leg and foot). • Memorize the main features of the – Bones of the thigh (femur & patella) – Bones of the leg (tibia & Fibula). – Bones of the foot (tarsals, metatarsals and phalanges) • Recognize the side of the bone BONES OF THIGH (Femur and Patella) Femur: . Articulates above with acetabulum of hip bone to form the hip joint. Articulates below with tibia and patella to form the knee joint. BONES OF THIGH (Femur and Patella) • Femur : Consists of : • Upper end • Shaft • Lower end Posterior view Anterior View UPPER END OF FEMUR • Head : • It articulates with acetabulum of hip bone to form hip joint. • Has a depression in the center (fovea capitis), for the attachment of ligament NECK of the head of femur. • Obturator artery passes along this ligament to supply head of femur. • Neck : • It connects head to the shaft. UPPER END OF FEMUR • Greater & lesser trochanters : • Anteriorly, connecting the 2 trochanters, the inter-trochanteric line, where the iliofemoral ligament is attached. • Posteriorly, the inter- trochanteric crest, on which is the quadrate tubercle (Qudratus femoris muscle). SHAFT OF FEMUR It has 3 surfaces Anterior Medial Lateral It has 3 borders Two rounded medial and lateral One thick posterior border or ridge called Anterior view Posterior view linea aspera SHAFT OF FEMUR • Anteriorly : is smooth and rounded. • Posteriorly : has a ridge, the linea aspera. • Posteriorly : below the greater trochanter is the gluteal tuberosity for attachment of gluteus M maximus muscle. -

EZ-IO PD Distal Tibial Access
® EZ-IO PD Distal Tibial Access ® Training Program The EZ-IO PD® was developed as a direct result of the need to improve intraosseous access in patients of smaller size. The images and training that follow were designed to simplify IO understanding and improve emergency vascular access. Our collective goal has long been Immediate, Safe and Effective vascular access for all critical patients. Vidacare’s approach to this goal is simple - the right equipment - in the best hands - where it’s needed most. At the completion of this program if you still have questions or concerns please call us at 1.866.479.8500 or visit our web site at www.vidacare.com. We at Vidacare® appreciate what you do and the time you devote to it. Thank you for inviting us to be a member of your team! 1 Indications for EZ-IO PD Access ¾ Altered level of consciousness ¾ Respiratory compromise ¾ Hemodynamic instability To gain immediate vascular access in an emergency FOR PATIENTS WEIGHTING BETWEEN 3 – 39 KILOGRAMS Listed here are the primary indications. Can you think of specific conditions that would fit each indications? Examples of disease states often meeting these criteria include, but are not limited to the following: Cardiac arrest, Status epilepticus, All shock states, Arrythmias, Dehydration Burns, Drug Overdose, DKA (diabetic), Renal failure, Stroke, AMI, Coma, OB complications, Thyroid crisis, Trauma, Anaphylaxis, CHF, Emphysema, Respiratory arrest, Hemophiliac crisis 2 Contraindications for EZ-IO PD Access ¾ Fracture (targeted bone) ¾ Previous orthopedic procedures near insertion site (IO within past 24 hours/Prosthetic Limb or joint) ¾ Infection at the insertion site ¾ Inability to locate landmarks or excessive tissue These are the contraindications.