Johnson & Johnson (JNJ)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faqs on COVID-19 Vaccine
COVID-19 Frequently Asked Questions COVID-19 Vaccines About COVID-19 Vaccines Q: Why is a COVID-19 vaccine needed if social distancing and wearing masks prevent the COVID-19 virus from spreading? A: Vaccines boost your immune system, so it will be ready to fight the virus if you are exposed. Vaccination combined with ongoing prevention efforts including wearing face masks that cover the mouth and nose, frequent hand washing and staying at least 6 feet away from others offer the best protection against COVID-19. Q: How many COVID-19 vaccines are available? Which one should I get? A: In the United States, three COVID-19 vaccines have been granted emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) and recommended for use by the Centers for Disease Control and Prevention (CDC). These vaccines, manufactured by Pfizer, Moderna, and Johnson & Johnson (Janssen), have all been proven safe and effective at preventing serious illness, hospitalization, and death from COVID-19 disease. The CDC recommends getting the first vaccine available to you for protection from COVID-19. While vaccine supply is limited, individuals likely will not get to choose which vaccine to receive, and will be given what is available from their vaccine provider at the time they receive their immunization. Q: Are all of the COVID-19 vaccines effective? A: Yes. COVID-19 vaccines from Pfizer, Moderna and Johnson & Johnson (Janssen) have been approved for emergency use by the FDA, and recommended for use by the CDC after a rigorous analysis proved their effectiveness. During studies, all the vaccines were shown to prevent serious illness from COVID-19 at high effectiveness rates. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Summary Analgesics Dec2019
Status as of December 31, 2019 UPDATE STATUS: N = New, A = Advanced, C = Changed, S = Same (No Change), D = Discontinued Update Emerging treatments for acute and chronic pain Development Status, Route, Contact information Status Agent Description / Mechanism of Opioid Function / Target Indication / Other Comments Sponsor / Originator Status Route URL Action (Y/No) 2019 UPDATES / CONTINUING PRODUCTS FROM 2018 Small molecule, inhibition of 1% diacerein TWi Biotechnology / caspase-1, block activation of 1 (AC-203 / caspase-1 inhibitor Inherited Epidermolysis Bullosa Castle Creek Phase 2 No Topical www.twibiotech.com NLRP3 inflamasomes; reduced CCP-020) Pharmaceuticals IL-1beta and IL-18 Small molecule; topical NSAID Frontier 2 AB001 NSAID formulation (nondisclosed active Chronic low back pain Phase 2 No Topical www.frontierbiotech.com/en/products/1.html Biotechnologies ingredient) Small molecule; oral uricosuric / anti-inflammatory agent + febuxostat (xanthine oxidase Gout in patients taking urate- Uricosuric + 3 AC-201 CR inhibitor); inhibition of NLRP3 lowering therapy; Gout; TWi Biotechnology Phase 2 No Oral www.twibiotech.com/rAndD_11 xanthine oxidase inflammasome assembly, reduced Epidermolysis Bullosa Simplex (EBS) production of caspase-1 and cytokine IL-1Beta www.arraybiopharma.com/our-science/our-pipeline AK-1830 Small molecule; tropomyosin Array BioPharma / 4 TrkA Pain, inflammation Phase 1 No Oral www.asahi- A (ARRY-954) receptor kinase A (TrkA) inhibitor Asahi Kasei Pharma kasei.co.jp/asahi/en/news/2016/e160401_2.html www.neurosmedical.com/clinical-research; -

Mcneil Consumer : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: MCNEIL CONSUMER : MDL NO. 2190 HEALTHCARE, ET AL., MARKETING : AND SALES PRACTICES LITIGATION : : Applies to: : ALL ACTIONS : MEMORANDUM McLaughlin, J. July 13, 2012 This multidistrict litigation arises out of quality control problems at the defendants’ facility manufacturing over- the-counter healthcare products in Fort Washington, Pennsylvania, which led to a series of recalls of those products. The named plaintiffs assert claims for economic loss on behalf of a putative nationwide class against Johnson & Johnson (“J&J”), McNeil Consumer Healthcare (“McNeil”), and four of their executives. The plaintiffs allege that they overpaid for the defendants’ products as a result of the recalls and the defendants’ scheme to conceal or downplay the scope of the quality control problems. The defendants, who have offered a coupon or cash refund to consumers who purchased recalled drugs, have moved to dismiss the operative complaint, and assert that the named plaintiffs lack constitutional standing and have not met the applicable pleading standard. The Court will grant the defendants’ motion because the plaintiffs have not pled facts that show a cognizable injury in fact, which is required to confer Article III standing. I. Procedural Background This litigation resulted from the consolidation of ten individual actions filed around the country. Haviland v. McNeil Consumer Healthcare, No. 10-2195, was filed in this Court on May 12, 2010, asserting economic injuries arising out of the April 30, 2010 recall of over-the-counter children’s drugs by McNeil, a part of the J&J “Family of Companies.” Eight additional cases, also arising out of the April 2010 recall, were filed in district courts around the country.1 All cases asserted claims for economic injury only, with the exception of Rivera v. -

Research & Development of Vaccines to Prevent SARS-Cov2 Infection
Monthly update Research & development of vaccines to prevent SARS-coV2 infection Updated May 2021 Disclaimer No vaccine against COVID-19 is approved. This document does not provide guidance on what vaccine or medicines to take. Please avoid self-prescription and always refer to your doctor before making any treatment decision. This document provides a selection of updates on the research and development of vaccines for the current coronavirus infection. Those highlights are for the information of patient organisations/ groups, advocates and people living with a rare disease. EURORDIS takes reasonable steps to verify the accuracy of the information presented. This document does not constitute, and shall not be deemed or construed as, any approval or endorsement by EURORDIS of such product or entity. Updated May 2021 Contents (click to navigate in document) A ‘must-read’ introduction .................................................................................................................................................................................................................................. 4 Resources ........................................................................................................................................................................................................................................................... 5 Vaccines in development ................................................................................................................................................................................................................................... -

Notice Under S66 of the Commerce Act 1986 Application by Johnson & Johnson to Acquire the Stock, Assets and Business Of
PUBLIC COPY Notice under s66 of the Commerce Act 1986 Application by Johnson & Johnson to acquire the stock, assets and business of the Consumer Healthcare division of Pfizer Inc. COMMERCE ACT 1986: BUSINESS ACQUISITION SECTION 66: NOTICE SEEKING CLEARANCE 28 September 2006 The Registrar Business Acquisitions and Authorisations Commerce Commission PO Box 2351 Wellington Pursuant to s66(1) of the Commerce Act 1986 notice is hereby given seeking clearance of a proposed business acquisition. 518689_1.DOC 2 CONTENTS EXECUTIVE SUMMARY PART 1: TRANSACTION DETAILS 1 The business acquisition for which clearance is sought 2 The person giving this notice 3 Confidentiality 4 Participants 5 Interconnected and associated persons 6 Beneficial interests 7 Links between participants 8 Common directorships 9 Business activities of the participants 10 Reasons for the proposed acquisition PART II: IDENTIFICATION OF MARKETS AFFECTED 11 Horizontal aggregation 12 Differentiated product markets 13 Differentiated product markets 14 Vertical integration 15 Other business acquisitions PARTS III, IV AND V: CONSTRAINTS ON MARKET POWERS BY EXISTING AND POTENTIAL COMPETITION AND OTHER POTENTIAL CONSTRAINTS 16 Allergy medication 17 Products for the treatment of worms 18 Thrush treatment CERTIFICATE APPENDICES 1. Heartburn and indigestion remedies (MYLANTA and MOTILIUM) 2. Worm treatments (COMBANTRIN, VERMOX) 3. Cold, flu, nasal decongestant, cough relief and sort throat medications (CODRAL, SINUTAB, SUDAFED, BENADRYL, BRONDECON) 4. Allergy relief products (ACTIFED, SINUTAB, SUDAFED, VISINE, LIVOSTIN) 518689_1.DOC 3 5. Thrush treatments (DIFLUCAN ONE, DAKTARIN, DAKTAGOLD, NIZORAL, SPORANOX) 6. Shampoo (PREGAINE, ROGAINE, NEUTROGENA, JOHNSON’S BABY SHAMPOO) 7. Hand hygiene (PURELL and MICRO SHIELD) 8. Competitor worm treatment products 9. Multinational pharmaceutical businesses: GlaxoSmithKline, Douglas Pharmaceuticals, Alphapharm, Bayer Group 10. -
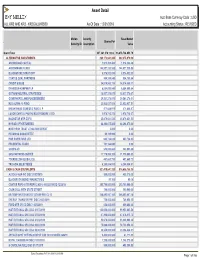
Asset Detail Acct Base Currency Code : USD ALL KR2 and KR3 - KR2GALLKRS00 As of Date : 12/31/2018 Accounting Status : REVISED
Asset Detail Acct Base Currency Code : USD ALL KR2 AND KR3 - KR2GALLKRS00 As Of Date : 12/31/2018 Accounting Status : REVISED Mellon Security Base Market . Shares/Par Security ID Description Value Grand Total 337,341,374,122.6.. 16,678,734,053.79 ALTERNATIVE INVESTMENTS 383,172,041.330 382,375,079.30 ANCHORAGE CAPITAL 1,974,352.480 1,974,352.48 ARROWMARK FUND I 144,927,230.580 144,927,230.58 BLACKSTONE STRAT OPP 8,576,532.030 8,576,532.03 COATUE QUAL PARTNERS 904,189.840 904,189.84 CREDIT SUISSE 34,074,860.110 34,074,860.11 DAVIDSON-KEMPNER LP 6,884,099.840 6,884,099.84 GOTHAM NEUTRAL STRATEGIES 18,837,376.670 18,837,376.67 GOVERNORS LANE FUNDÉÉÉÉÉÉÉÉ 29,561,278.010 29,561,278.01 H2O ALPHA-10 FUND 23,932,037.510 23,932,037.51 KNIGHTHEAD DOMESTIC FUND L P 471,648.970 471,648.97 LUXOR CAPITAL PARTNERS OFFSHORE LTDD 2,978,710.270 2,978,710.27 MAGNETAR MTP EOF II 25,879,631.530 25,879,631.53 MYRIAD OPPORTUNITIES 64,294,675.000 64,294,675.00 NORTHERN TRUST LITIGATION CREDIT 2.000 0.00 PERSHING SQUAREÉÉÉÉ 95,795.990 0.00 PINE RIVER FUND LTD 603,724.620 603,724.62 PRUDENTIAL FUND I 701,164.040 0.00 SCOPIA VII 548,095.880 548,095.88 SRS PARTNERS USÉÉÉÉ 11,179,090.320 11,179,090.32 TOURBILLON GLOBAL EQ 491,660.730 491,660.73 TRICADIA SELECTÉÉÉÉ 6,255,884.910 6,255,884.91 CASH & CASH EQUIVALENTS 927,419,647.320 916,686,158.35 AUTOLIV ASP INC DISC 01/02/2019 500,000.000 499,375.00 BLACKROCK MONEY MARKET FD B 90.160 90.16 CANTOR REPO A TRI REPO 2.450% 01/02/2019 DD 12/28/18 295,700,000.000 295,700,000.00 CASH COLL WITH STATE STREET 190,000.000 190,000.00 -

Johnson & Johnson's Janssen COVID-19 Vaccine Info
Johnson & Johnson’s Janssen COVID-19 Vaccine: Is It Efficacious? Purpose There has been observed brand hesitancy between the Johnson & Johnson Janssen COVID-19 vaccine as compared to the Pfizer and Moderna COVID-19 vaccine where individuals have expressed concerns over disparities in efficacy. This likely stems from the blanket efficacy statements reported in media outlets where the Johnson & Johnson’s vaccine reports an efficacy of 72% compared to 94% & 95% for Moderna and Pfizer respectively. The purpose of this info-bite is to clarify the concerns over efficacy so we can reassure our patients that all three vaccination options are efficacious and safe. The goal is to reduce vaccine brand hesitancy, as it may lead to a delay in vaccination, which may put our patients at risk. Is the Johnson & Johnson’s Janssen vaccine efficacious? Yes, the Johnson & Johnson Janssen vaccine has proven efficacy. Its reportedly 100% efficacious against death due to COVID-19 AND prevention against hospitalization. Additionally, it is potentially effective against variant strains as it maintained high efficacy against severe disease (73-82%) across world regions. The 72% efficacy often noted includes prevention of mild & moderate disease; it is unclear at this time how efficacious the vaccine is against mild & moderate disease. Is the Johnson & Johnson’s Janssen vaccine less efficacious than the Pfizer and Moderna vaccine? There is no evidence to claim that any of the three vaccines available are more efficacious than another. None of these vaccines have been compared in a head-to-head trial. Additionally, since the vaccine trials were tested at different times and locations, it further reduces one’s ability to claim one is better than the other. -

Nevada Medicaid Formulary
Nevada Medicaid-Approved Preferred Drug List Effective August 15, 2021 Legend In each class, drugs are listed alphabetically by either brand name or generic name. Brand name drug: Uppercase in bold type Generic drug: Lowercase in plain type AL: Age Limit Restrictions DO: Dose Optimization Program GR: Gender Restriction OTC: Over the counter medication available with a prescription. (Prescribers please indicate OTC on the prescription) PA: Prior authorization is required. Prior authorization is the process of obtaining approval of benefits before certain prescriptions are filled. QL: Quantity limits; certain prescription medications have specific quantity limits per prescription or per month. SP: Specialty Pharmacy ST: Step therapy is required. You may need to use one medication before benefits for the use of another medication can be authorized. Drug Name Reference Notes *ADHD/ANTI-NARCOLEPSY/ANTI- OBESITY/ANOREXIANTS* *ADHD AGENT - SELECTIVE ALPHA ADRENERGIC AGONISTS*** clonidine hcl er oral tablet extended release Kapvay AL; QL 12 hour *ADHD AGENT - SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR*** atomoxetine hcl oral capsule Strattera DO; AL; QL *AMPHETAMINE MIXTURES*** amphetamine-dextroamphet er oral capsule extended release 24 hour 10 mg, 15 mg, 5 Adderall XR DO; AL; QL mg amphetamine-dextroamphet er oral capsule extended release 24 hour 20 mg, 25 mg, 30 Adderall XR AL; QL mg amphetamine-dextroamphetamine oral Adderall DO; AL; QL tablet 10 mg, 12.5 mg, 15 mg, 5 mg, 7.5 mg amphetamine-dextroamphetamine oral Adderall AL tablet 20 mg, -

Respirerx Pharmaceuticals Inc. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K [X] Annual Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, 2018 OR [ ] Transition Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Commission file number 1-16467 RespireRx Pharmaceuticals Inc. (Exact name of registrant as specified in its charter) Delaware 33-0303583 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification Number) 126 Valley Road, Suite C Glen Rock, New Jersey 07452 (Address of principal executive offices, including zip code) (201) 444-4947 (Registrant’s telephone number, including area code) Securities registered under Section 12(b) of the Act: None Securities registered under Section 12(g) of the Act: Common Stock, $0.001 par value (Title of Class) Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES [ ] NO [X] Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. YES [ ] NO [X] Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports); and (2) has been subject to such filing requirements for the past 90 days. YES [X] NO [ ] Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). -

Swiss Biotech Report – "Agility, Leadership and Innovation in the Time of COVID-19” – Is Indeed Appropriate
SwissSwiss BiotechBiotech ReportReport 2021 2019Agility, leadership and innovation in the time of COVID-19 Schweizerische Eidgenossenschaft Confédération suisse Confederazione Svizzera Confederaziun svizra Swiss Confederation Innosuisse – Swiss Innovation Agency “The biopharma industry’s response to COVID-19 has truly been a global one – with Swiss companies and academic institutions at the forefront. Swiss-based biopharma players are contributing across the spectrum of patient needs.” George Scangos | Vir Biotechnology, Inc. “2020 was the best year ever for Swiss biotech in relation to financing activities, with a total of approximately CHF 3.4B raised” Jürg Zürcher | GSA Biotechnology Leader, EY “Compared to 2019, trading volumes rose sharply by 177% and the total free float market capitalization of all SIX-listed biotech companies was up 30% on 2020.” Fabian Gerber | SIX Agility, leadership and innovation in the time of COVID-19 “Technologies developed by scientists in Switzerland have been crucial in the fight against COVID-19, including cloning the virus, cryo electron microscopy to visualize the spike proteins, and the use of radioactive molecules that bind to receptor proteins to investigate how the virus enters the host.” Florian Fisch | Swiss National Science Foundation “On May 1 2020, Moderna and Lonza announced a strategic collaboration to enable manufacturing of up to 1 billion doses of the vaccine per year. Lonza acquired equipment and built the production lines in just 8 months.” Jan Lucht | scienceindustries “Industrial -

Janssen COVID-19 Vaccine Storage and Handling Summary
Janssen COVID-19 Vaccine (Johnson & Johnson) Storage and Handling Summary Basics Store vaccine in a refrigerator. See guidance below for further details. Check and record storage unit temperatures each workday. See guidance below for each type of temperature monitoring device. Save storage records for 3 years, unless your jurisdiction requires a longer time period. Deliveries Vaccine 1. The vaccine will arrive in a 2° to 8°C (36°F and 46°F) qualified shipping container. 2. Examine the shipment for signs of damage. 3. As a backup to the qualified shipping container, temperature monitors are placed in each shipment. Janssen vaccines may include thawed and frozen/partially frozen vaccines and due to this, only warm monitors will be included in Janssen shipments. 4. Remove the instruction card for the temperature monitor immediately. Follow the guide on the back of the card to read the monitor. 5. The expiration date is NOT printed on the vaccine vial or carton. To determine the expiration date: Scan the QR code on the outer carton, or Call 1-800-565-4008, or Visit www.vaxcheck.jnj. Write the expiration date on the carton. Ancillary Supply Kit An ancillary supply kit will be provided and includes enough supplies to administer 100 doses of vaccine. Administration supplies include needles, syringes, sterile alcohol prep pads, vaccination record cards (shot cards), and some PPE. The kit is delivered separately from the vaccine. Unpack the kit and check for receipt of the correct administration supplies and quantities. 1 08/24/2021 CS322139-C Janssen COVID-19 Vaccine (Johnson & Johnson) Storage and Handling Summary Refrigerator Storage CDC recommends storing vaccine between 2°C and Do not freeze.