Calcaneal Proportions in Primates and Locomotor Inferences in Anchomomys and Other Palaeogene Euprimates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming
Distribution and Stratigraphip Correlation of Upper:UB_ • Ju Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming U,S. GEOLOGICAL SURVEY PROFESS IONAL PAPER 1540 Cover. A member of the American Museum of Natural History 1896 expedition enter ing the badlands of the Willwood Formation on Dorsey Creek, Wyoming, near what is now U.S. Geological Survey fossil vertebrate locality D1691 (Wardel Reservoir quadran gle). View to the southwest. Photograph by Walter Granger, courtesy of the Department of Library Services, American Museum of Natural History, New York, negative no. 35957. DISTRIBUTION AND STRATIGRAPHIC CORRELATION OF UPPER PALEOCENE AND LOWER EOCENE FOSSIL MAMMAL AND PLANT LOCALITIES OF THE FORT UNION, WILLWOOD, AND TATMAN FORMATIONS, SOUTHERN BIGHORN BASIN, WYOMING Upper part of the Will wood Formation on East Ridge, Middle Fork of Fifteenmile Creek, southern Bighorn Basin, Wyoming. The Kirwin intrusive complex of the Absaroka Range is in the background. View to the west. Distribution and Stratigraphic Correlation of Upper Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Tatman Formations, Southern Bighorn Basin, Wyoming By Thomas M. Down, Kenneth D. Rose, Elwyn L. Simons, and Scott L. Wing U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1540 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1994 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Robert M. Hirsch, Acting Director For sale by U.S. Geological Survey, Map Distribution Box 25286, MS 306, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. -

Additional Fossil Evidence on the Differentiation of the Earliest Euprimates (Omomyidae/Adapidae/Steinius/Primate Evolution) KENNETH D
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 98-101, January 1991 Evolution Additional fossil evidence on the differentiation of the earliest euprimates (Omomyidae/Adapidae/Steinius/primate evolution) KENNETH D. ROSE* AND THOMAS M. BOWNt *Department of Cell Biology and Anatomy, The Johns Hopkins University School of Medicine, Baltimore, MD 21205; and tU.S. Geological Survey, Paleontology and Stratigraphy Branch, Denver, CO 80225 Communicated by Elwyn L. Simons, October 1. 1990 ABSTRACT Several well-preservedjaws ofthe rare North Table 1. U.S. Geological Survey (USGS) samples American omomyid primate Steinius vespertinus, including the Finder of first known antemolar dentitions, have been discovered in 1989 USGS no. Description sample and 1990 in the early Eocene Willwood Formation of the Bighorn Basin, Wyoming. They indicate that its dental formula 25026 Right dentary with P4-M3 S. J. Senturia is as primitive as those in early Eocene Donrussellia (Adapidae) and anterior alveoli and Teilhardina (Omomyidae)-widely considered to be the 25027 Right dentary with P3-M3 M. Shekelle most primitive known euprimates-and that in various dental 25028 Left dentary with P3-P4 J. J. Rose characters Steinius is as primitive or more so than Teilhardina. 28325 Left dentary with P3-P4 H. H. Covert Therefore, despite its occurrence at least 2 million years later and anterior alveoli than Teilhardina, S. vespertinus is the most primitive known 28326 Left dentary with P3-M1 T. M. Bown omomyid and one of the most primitive known euprimates. Its 28466 Isolated right M2 primitive morphology further diminishes the dental distinc- 28472 Isolated left M1 tions between Omomyidae and Adapidae at the beginning ofthe 28473 Isolated right P4 euprimate radiation. -

Fossil Primates
AccessScience from McGraw-Hill Education Page 1 of 16 www.accessscience.com Fossil primates Contributed by: Eric Delson Publication year: 2014 Extinct members of the order of mammals to which humans belong. All current classifications divide the living primates into two major groups (suborders): the Strepsirhini or “lower” primates (lemurs, lorises, and bushbabies) and the Haplorhini or “higher” primates [tarsiers and anthropoids (New and Old World monkeys, greater and lesser apes, and humans)]. Some fossil groups (omomyiforms and adapiforms) can be placed with or near these two extant groupings; however, there is contention whether the Plesiadapiformes represent the earliest relatives of primates and are best placed within the order (as here) or outside it. See also: FOSSIL; MAMMALIA; PHYLOGENY; PHYSICAL ANTHROPOLOGY; PRIMATES. Vast evidence suggests that the order Primates is a monophyletic group, that is, the primates have a common genetic origin. Although several peculiarities of the primate bauplan (body plan) appear to be inherited from an inferred common ancestor, it seems that the order as a whole is characterized by showing a variety of parallel adaptations in different groups to a predominantly arboreal lifestyle, including anatomical and behavioral complexes related to improved grasping and manipulative capacities, a variety of locomotor styles, and enlargement of the higher centers of the brain. Among the extant primates, the lower primates more closely resemble forms that evolved relatively early in the history of the order, whereas the higher primates represent a group that evolved more recently (Fig. 1). A classification of the primates, as accepted here, appears above. Early primates The earliest primates are placed in their own semiorder, Plesiadapiformes (as contrasted with the semiorder Euprimates for all living forms), because they have no direct evolutionary links with, and bear few adaptive resemblances to, any group of living primates. -

Primates, Adapiformes) Skull from the Uintan (Middle Eocene) of San Diego County, California
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 98:447-470 (1995 New Notharctine (Primates, Adapiformes) Skull From the Uintan (Middle Eocene) of San Diego County, California GREGG F. GUNNELL Museum of Paleontology, University of Michigan, Ann Arbor, Michigan 481 09-1079 KEY WORDS Californian primates, Cranial morphology, Haplorhine-strepsirhine dichotomy ABSTRACT A new genus and species of notharctine primate, Hespero- lemur actius, is described from Uintan (middle Eocene) aged rocks of San Diego County, California. Hesperolemur differs from all previously described adapiforms in having the anterior third of the ectotympanic anulus fused to the internal lateral wall of the auditory bulla. In this feature Hesperolemur superficially resembles extant cheirogaleids. Hesperolemur also differs from previously known adapiforms in lacking bony canals that transmit the inter- nal carotid artery through the tympanic cavity. Hesperolemur, like the later occurring North American cercamoniine Mahgarita steuensi, appears to have lacked a stapedial artery. Evidence from newly discovered skulls ofNotharctus and Smilodectes, along with Hesperolemur, Mahgarita, and Adapis, indicates that the tympanic arterial circulatory pattern of these adapiforms is charac- terized by stapedial arteries that are smaller than promontory arteries, a feature shared with extant tarsiers and anthropoids and one of the character- istics often used to support the existence of a haplorhine-strepsirhine dichot- omy among extant primates. The existence of such a dichotomy among Eocene primates is not supported by any compelling evidence. Hesperolemur is the latest occurring notharctine primate known from North America and is the only notharctine represented among a relatively diverse primate fauna from southern California. The coastal lowlands of southern California presumably served as a refuge area for primates during the middle and later Eocene as climates deteriorated in the continental interior. -

Evolutionary History of Lorisiform Primates
Evolution: Reviewed Article Folia Primatol 1998;69(suppl 1):250–285 oooooooooooooooooooooooooooooooo Evolutionary History of Lorisiform Primates D. Tab Rasmussen, Kimberley A. Nekaris Department of Anthropology, Washington University, St. Louis, Mo., USA Key Words Lorisidae · Strepsirhini · Plesiopithecus · Mioeuoticus · Progalago · Galago · Vertebrate paleontology · Phylogeny · Primate adaptation Abstract We integrate information from the fossil record, morphology, behavior and mo- lecular studies to provide a current overview of lorisoid evolution. Several Eocene prosimians of the northern continents, including both omomyids and adapoids, have been suggested as possible lorisoid ancestors, but these cannot be substantiated as true strepsirhines. A small-bodied primate, Anchomomys, of the middle Eocene of Europe may be the best candidate among putative adapoids for status as a true strepsirhine. Recent finds of Eocene primates in Africa have revealed new prosimian taxa that are also viable contenders for strepsirhine status. Plesiopithecus teras is a Nycticebus- sized, nocturnal prosimian from the late Eocene, Fayum, Egypt, that shares cranial specializations with lorisoids, but it also retains primitive features (e.g. four premo- lars) and has unique specializations of the anterior teeth excluding it from direct lorisi- form ancestry. Another unnamed Fayum primate resembles modern cheirogaleids in dental structure and body size. Two genera from Oman, Omanodon and Shizarodon, also reveal a mix of similarities to both cheirogaleids and anchomomyin adapoids. Resolving the phylogenetic position of these Africa primates of the early Tertiary will surely require more and better fossils. By the early to middle Miocene, lorisoids were well established in East Africa, and the debate about whether these represent lorisines or galagines is reviewed. -

Effects of Additional Postcranial Data on the Topologies of Early Eocene Primate
Effects of Additional Postcranial Data on the Topologies of Early Eocene Primate Phylogenies Julia Stone ANT 679HB Special Honors in the Department of Anthropology The University of Texas at Austin May 2021 Liza J. Shapiro Department of Anthropology Supervising Professor E. Christopher Kirk Department of Anthropology Second Reader Acknowledgements This project would not have been completed without the help of my thesis supervisor Dr. Liza Shapiro. I was inspired to start the project after taking her courses in Primate Evolution and Primate Anatomy. She also met with me many times throughout this year to discuss the trajectory of the project as well as discuss papers that became the foundation of the thesis. She assisted me through technology issues, unresolved trees, and many drafts, and she provided invaluable guidance throughout this process. I also want to thank Dr. Christopher Kirk for being my second reader and providing extremely helpful insight into how to write about phylogenetic results. He also helped me to be more specific and accurate in all of the sections of the thesis. I learned a lot about writing theses in general due to his comments on my drafts. I also truly appreciate the help of Ben Rodwell, a graduate student at the University of Texas at Austin. He provided excellent help and support regarding the Mesquite and TNT software used in this project. ii Effects of Additional Postcranial Data on the Topologies of Early Eocene Primate Phylogenies by Julia Stone, BA The University of Texas at Austin SUPERVISOR: Liza J. Shapiro There are multiple hypotheses regarding the locomotor behaviors of the last common ancestor to primates and which fossil primates best represent that ancestor. -
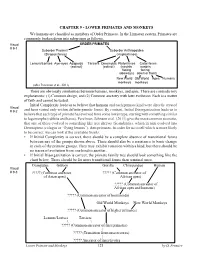
CHAPTER 9 - LOWER PRIMATES and MONKEYS We Humans Are Classified As Members of Order Primates
CHAPTER 9 - LOWER PRIMATES AND MONKEYS We humans are classified as members of Order Primates. In the Linnaean system, Primates are commonly broken down into subgroups as follows. Visual ORDER PRIMATES # 9-1 Suborder Prosimii Suborder Anthropoidea (Strepsirrhines) (Haplorrhines) Lemurs/Lorises Aye-ayes Adapoids Tarsiers Omomyids Platyrrhines Catarrhines (extinct) (extinct) (nostrils nostrils facing facing sideways) down or front) New World Old World Apes Humans monkeys monkeys (after Perelman et al., 2011) There are obviously similarities between humans, monkeys, and apes. There are contradictory explanations: (1) Common design, and (2) Common ancestry with later evolution. Each is a matter of faith and cannot be tested. Initial Complexity leads us to believe that humans and each primate kind were directly created Visual # 9-2 and have varied only within definite genetic limits. By contrast, Initial Disorganization leads us to believe that each type of primate has evolved from some lower type, starting with something similar to lagomorphs (rabbits and hares). Perelman, Johnson et al. (2011) give the most common scenario, that one of these evolved to something like tree shrews (Scandentia), which in turn evolved into Dermoptera (colugos or “flying lemurs”), then primates. In order for us to tell which is more likely to be correct, we can look at the available fossils. • If Initial Complexity is correct, there should be a complete absence of transitional forms between any of the groups shown above. There should also be a resistance to basic change in each of the primate groups. They may exhibit variation within a kind, but there should be no traces of evolution from one kind to another. -

Science Journals — AAAS
RESEARCH ◥ differ from those of Wailekia and Kyitchaungia RESEARCH ARTICLES in having lingually open trigonids with proto- conid and metaconid more closely approximated and talonids bearing more trenchant cristids. PRIMATE EVOLUTION UppermolarsdifferfromthoseofGuangxilemur in having more prominent parastyles and pre- and postprotocristae joining the bases of the Oligocene primates from China reveal paracone and metacone, respectively, rather than merging with the pre- and postcingula. Upper molars with weaker buccal cingulum and incom- divergence between African and plete mesostyle, in contrast to Guangxilemur tongi. Lower molars differ from those of Guang- Asian primate evolution xilemur singsilai in having lingually open trig- onids and deeper, more extensive valley separating Xijun Ni,1,2* Qiang Li,1,2 Lüzhou Li,1 K. Christopher Beard3,4 entoconid and hypoconulid. Differs from Miocene 4 sivaladapids in lacking fully molariform P4 and P Profound environmental and faunal changes are associated with climatic deterioration and having upper molars with distinct pericone during the Eocene-Oligocene transition (EOT) roughly 34 million years ago. Reconstructing and hypocone cusps and pre- and postprotocris- how Asian primates responded to the EOT has been hindered by a sparse record of tae joining bases of paracone and metacone. Oligocene primates on that continent. Here, we report the discovery of a diverse primate Etymology: Generic name recognizes the geo- fauna from the early Oligocene of southern China. In marked contrast to Afro-Arabian graphic provenance of this taxon and its adapi- Downloaded from Oligocene primate faunas, this Asian fauna is dominated by strepsirhines. There appears to form affinities. be a strong break between Paleogene and Neogene Asian anthropoid assemblages. -

Vertical Support Use and Primate Origins Gabriel S
www.nature.com/scientificreports OPEN Vertical support use and primate origins Gabriel S. Yapuncich 1, Henry J. Feng1, Rachel H. Dunn2, Erik R. Seifert 3 & Doug M. Boyer1 Adaptive scenarios of crown primate origins remain contentious due to uncertain order of acquisition Received: 1 May 2019 and functional signifcance of the clade’s diagnostic traits. A feature of the talus bone in the ankle, Accepted: 6 August 2019 known as the posterior trochlear shelf (PTS), is well-regarded as a derived crown primate trait, but Published: xx xx xxxx its adaptive signifcance has been obscured by poorly understood function. Here we propose a novel biomechanical function for the PTS and model the talus as a cam mechanism. By surveying a large sample of primates and their closest relatives, we demonstrate that the PTS is most strongly developed in extant taxa that habitually grasp vertical supports with strongly dorsifexed feet. Tali of the earliest fossils likely to represent crown primates exhibit more strongly developed PTS cam mechanisms than extant primates. As a cam, the PTS may increase grasping efciency in dorsifexed foot postures by increasing the path length of the fexor fbularis tendon, and thus improve the muscle’s ability to maintain fexed digits without increasing energetic demands. Comparisons are made to other passive digital fexion mechanisms suggested to exist in other vertebrates. These results provide robust anatomical evidence that the habitual vertical support use exerted a strong selective pressure during crown primate origins. Te talus is an important element for reconstructing positional behavior throughout primate evolution because the bone’s morphology correlates well with locomotor and postural behaviors of living euarchontans (the mam- malian clade including Primates, Scandentia, Dermoptera) and it is frequently preserved in fossil assemblages1–4. -

A Fossil Primate of Uncertain Affinities from the Earliest Late Eocene of Egypt
A fossil primate of uncertain affinities from the earliest late Eocene of Egypt Erik R. Seifferta,1, Elwyn L. Simonsb,1, Doug M. Boyerc, Jonathan M. G. Perryd, Timothy M. Ryane, and Hesham M. Sallamf aDepartment of Anatomical Sciences, Stony Brook University, Stony Brook, NY 11794-8081; bDivision of Fossil Primates, Duke Lemur Center, Durham, NC 27705; cDepartment of Ecology and Evolution, Stony Brook University, Stony Brook, NY 11794-5245; dDepartment of Anatomy, Midwestern University, Downers Grove, IL 60515; eDepartment of Anthropology, Pennsylvania State University, University Park, PA 16802; and fDepartment of Earth Sciences, University of Oxford, Oxford OX1 3PR, United Kingdom Contributed by Elwyn L. Simons, February 18, 2010 (sent for review January 5, 2010) Paleontological work carried out over the last 3 decades has es- as caenopithecines are likely derived from an independent colo- tablished that three major primate groups were present in the Eocene nization from Europe or Asia. The timing of anthropoids’ arrival in of Africa—anthropoids, adapiforms, and advanced strepsirrhines. Afro-Arabia is unclear, but the presence of multiple anthropoid Here we describe isolated teeth of a previously undocumented pri- species at BQ-2 supports a colonization in the late middle Eocene mate from the earliest late Eocene (≈37 Ma) of northern Egypt, Nos- or earlier. mips aenigmaticus, whose phylogenetic placement within Primates is Here we describe a rare and enigmatic primate species from BQ-2 unclear. Nosmips is smaller than the sympatric adapiform Afradapis that, unlike other primates from theEoceneofAfrica,doesnotfit but is considerably larger than other primate taxa known from the unambiguously into either Anthropoidea or Strepsirrhini on the basis same paleocommunity.