Alterations in Eukaryotic Elongation Factor Complex Proteins (Eef1s) in Cancer and Their Implications in Epigenetic Regulation T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of California, San Diego
UNIVERSITY OF CALIFORNIA, SAN DIEGO The post-terminal differentiation fate of RNAs revealed by next-generation sequencing A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Biomedical Sciences by Gloria Kuo Lefkowitz Committee in Charge: Professor Benjamin D. Yu, Chair Professor Richard Gallo Professor Bruce A. Hamilton Professor Miles F. Wilkinson Professor Eugene Yeo 2012 Copyright Gloria Kuo Lefkowitz, 2012 All rights reserved. The Dissertation of Gloria Kuo Lefkowitz is approved, and it is acceptable in quality and form for publication on microfilm and electronically: __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ Chair University of California, San Diego 2012 iii DEDICATION Ma and Ba, for your early indulgence and support. Matt and James, for choosing more practical callings. Roy, my love, for patiently sharing the ups and downs of this journey. iv EPIGRAPH It is foolish to tear one's hair in grief, as though sorrow would be made less by baldness. ~Cicero v TABLE OF CONTENTS Signature Page .............................................................................................................. iii Dedication .................................................................................................................... -

Evolution of Translation EF-Tu: Trna
University of Illinois at Urbana-Champaign Luthey-Schulten Group NIH Resource for Macromolecular Modeling and Bioinformatics Computational Biophysics Workshop Evolution of Translation EF-Tu: tRNA VMD Developer: John Stone MultiSeq Developers Tutorial Authors Elijah Roberts Ke Chen John Eargle John Eargle Dan Wright Zhaleh Ghaemi Jonathan Lai Zan Luthey-Schulten August 2014 A current version of this tutorial is available at http://www.scs.illinois.edu/~schulten/tutorials/ef-tu CONTENTS 2 Contents 1 Introduction 3 1.1 The Elongation Factor Tu . 3 1.2 Getting Started . 4 1.2.1 Requirements . 4 1.2.2 Copying the tutorial files . 4 1.2.3 Working directory . 4 1.2.4 Preferences . 4 1.3 Configuring BLAST for MultiSeq . 5 2 Comparative Analysis of EF-Tu 5 2.1 Finding archaeal EF1A sequences . 6 2.2 Aligning archaeal sequences and removing redundancy . 8 2.3 Finding bacteria EF-Tu sequences . 11 2.4 Performing ClustalW Multiple Sequence and Profile-Profile Align- ments . 12 2.5 Creating Multiple Sequence with MAFFT . 16 2.6 Conservation of EF-Tu among the Bacteria . 16 2.7 Finding conserved residues across the bacterial and archaeal do- mains . 20 2.8 EF-Tu Interface with the Ribosome . 21 3 Computing a Maximum Likelihood Phylogenetic Tree with RAxML 23 3.1 Load the Phylogenetic Tree into MultiSeq . 25 3.2 Reroot and Manipulate the Phylogenetic Tree . 25 4 MultiSeq TCL Scripting: Genomic Context 27 5 Appendix A 30 5.1 Building a BLAST Database . 30 6 Appendix B 31 6.1 Saving QR subset of alignments in PHYLIP and FASTA format 31 6.2 Calculating Maximum Likelihood Trees with RAxML . -

Allosteric Collaboration Between Elongation Factor G and the Ribosomal L1 Stalk Directs Trna Movements During Translation
Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation Jingyi Feia, Jonathan E. Bronsona, Jake M. Hofmanb,1, Rathi L. Srinivasc, Chris H. Wigginsd and Ruben L. Gonzalez, Jr.a,2 aDepartment of Chemistry, bDepartment of Physics, cThe Fu Foundation School of Engineering and Applied Science and dDepartment of Applied Physics and Applied Mathematics, Columbia University, New York, NY 10027 1Current address: Yahoo! Research, 111 West 40th Street, 17th Floor, NewYork, NY 10018 2To whom correspondence may be addressed. E-mail: [email protected] Classification: Biological Sciences, Biochemistry ABSTRACT Determining the mechanism by which transfer RNAs (tRNAs) rapidly and precisely transit through the ribosomal A, P and E sites during translation remains a major goal in the study of protein synthesis. Here, we report the real-time dynamics of the L1 stalk, a structural element of the large ribosomal subunit that is implicated in directing tRNA movements during translation. Within pre-translocation ribosomal complexes, the L1 stalk exists in a dynamic equilibrium between open and closed conformations. Binding of elongation factor G (EF-G) shifts this equilibrium towards the closed conformation through one of at least two distinct kinetic mechanisms, where the identity of the P-site tRNA dictates the kinetic route that is taken. Within post-translocation complexes, L1 stalk dynamics are dependent on the presence and identity of the E-site tRNA. Collectively, our data demonstrate that EF-G and the L1 stalk allosterically collaborate to direct tRNA translocation from the P to the E sites, and suggest a model for the release of E-site tRNA. -

Eef2k) Natural Product and Synthetic Small Molecule Inhibitors for Cancer Chemotherapy
International Journal of Molecular Sciences Review Progress in the Development of Eukaryotic Elongation Factor 2 Kinase (eEF2K) Natural Product and Synthetic Small Molecule Inhibitors for Cancer Chemotherapy Bin Zhang 1 , Jiamei Zou 1, Qiting Zhang 2, Ze Wang 1, Ning Wang 2,* , Shan He 1 , Yufen Zhao 2 and C. Benjamin Naman 1,* 1 Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Research Center, College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo 315800, China; [email protected] (B.Z.); [email protected] (J.Z.); [email protected] (Z.W.); [email protected] (S.H.) 2 Institute of Drug Discovery Technology, Ningbo University, Ningbo 315211, China; [email protected] (Q.Z.); [email protected] (Y.Z.) * Correspondence: [email protected] (N.W.); [email protected] (C.B.N.) Abstract: Eukaryotic elongation factor 2 kinase (eEF2K or Ca2+/calmodulin-dependent protein kinase, CAMKIII) is a new member of an atypical α-kinase family different from conventional protein kinases that is now considered as a potential target for the treatment of cancer. This protein regulates the phosphorylation of eukaryotic elongation factor 2 (eEF2) to restrain activity and inhibit the elongation stage of protein synthesis. Mounting evidence shows that eEF2K regulates the cell cycle, autophagy, apoptosis, angiogenesis, invasion, and metastasis in several types of cancers. The Citation: Zhang, B.; Zou, J.; Zhang, expression of eEF2K promotes survival of cancer cells, and the level of this protein is increased in Q.; Wang, Z.; Wang, N.; He, S.; Zhao, many cancer cells to adapt them to the microenvironment conditions including hypoxia, nutrient Y.; Naman, C.B. -

The Role of Myc-Induced Protein Synthesis in Cancer
Published OnlineFirst November 24, 2009; DOI: 10.1158/0008-5472.CAN-09-1970 Review The Role of Myc-Induced Protein Synthesis in Cancer Davide Ruggero School of Medicine and Department of Urology, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, California Abstract tions and processing by directly controlling the expression of ribo- Deregulation in different steps of translational control is an nucleases, rRNA-modifying enzymes, and nucleolar proteins NPM emerging mechanism for cancer formation. One example of involved in ribosome biogenesis such as nucleophosmin ( ), Nop52, Nop56 DKC1 an oncogene with a direct role in control of translation is , and (Table 1;ref. 16). Furthermore, Myc in- UBF the Myc transcription factor. Myc directly increases protein duces Upstream Binding Factor ( ) expression, which is an es- synthesis rates by controlling the expression of multiple com- sential transcription factor for RNA Pol I-mediated transcription ponents of the protein synthetic machinery, including ribo- (21). It has also been recently shown that a fraction of the Myc somal proteins and initiation factors of translation, Pol III protein is localized in the nucleolus and directly regulates rRNA rDNA and rDNA. However, the contribution of Myc-dependent in- synthesis by binding to E-box elements located in the pro- – creases in protein synthesis toward the multistep process moter (17 19). In addition, it can activate Pol I transcription by rDNA leading to cancer has remained unknown. Recent evidence binding and recruiting to the promoter SL1, which is essen- strongly suggests that Myc oncogenic signaling may monopo- tial for the assembly of the RNA Pol I pre-initiation complex (18). -

Supplementary Information
SUPPLEMENTARY INFORMATION Myeloperoxidase-derived 2-chlorohexadecanal is generated in mouse heart during endotoxemia and induces modification of distinct cardiomyocyte protein subsets in vitro Jürgen Prasch, Eva Bernhart, Helga Reicher, Manfred Kollroser, Gerald N. Rechberger, Chintan N. Koyani, Christopher Trummer, Lavinia Rech, Peter P. Rainer, Astrid Hammer, Ernst Malle, Wolfgang Sattler Table S1: Biological process gene ontology (GO) enrichment analysis. #term ID term description observed background false discovery matching proteins in network (labels) gene count gene count rate GO:0006457 protein folding 10 153 5.21e-09 Cct3,Cct5,Cct8,Fkbp4,Hsp90aa1,Hsp a1l,Hspb1,Pdia3,Pdia6,Tcp1 GO:0007339 binding of sperm to 6 36 4.02e-07 Aldoa,Cct3,Cct5,Cct8,Hspa1l,Tcp1 zona pellucida GO:0061077 chaperone-mediated 6 60 2.67e-06 Cct3,Cct5,Cct8,Fkbp4,Hspb1,Tcp1 protein folding GO:0017144 drug metabolic process 11 494 4.06e-06 Aldh2,Aldoa,Eno1,Gapdh,Hsp90aa1,I dh3a,Ldha,Ndufs2,Pgam1,Phgdh,Uq crc1 GO:2000573 positive regulation of 6 69 4.16e-06 Cct3,Cct5,Cct8,Ddx39b,Hsp90aa1,Tc DNA biosynthetic p1 process GO:0009987 cellular process 47 12459 4.22e-06 Alad,Alb,Aldh2,Aldoa,Cct3,Cct5,Cct8, Dctn2,Ddx39,Ddx39b,Des,Eef1g,Eef 2,Eif3f,Eif4a2,Eno1,Fdps,Fkbp4,Gap dh,Hnrnpl,Hsp90aa1,Hspa1l,Hspb1,I dh3a,Ldha,Lmna,Lyz1,Ndufs2,Pcna, Pdia3,Pdia6,Pgam1,Phgdh,Prph,Psm d13,Rpsa,Ruvbl2,Tcp1,Tuba3b,Tubal 3,Tubb3,Tubb6,Uap1l1,Uqcrc1,Uqcrc 2,Vim,Ywhab GO:1904851 positive regulation of 4 10 4.22e-06 Cct3,Cct5,Cct8,Tcp1 establishment of protein localization to telomere GO:0046031 -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement. -

Ef-G:Trna Dynamics During the Elongation Cycle of Protein Synthesis
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2015 Ef-G:trna Dynamics During the Elongation Cycle of Protein Synthesis Rong Shen University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biochemistry Commons Recommended Citation Shen, Rong, "Ef-G:trna Dynamics During the Elongation Cycle of Protein Synthesis" (2015). Publicly Accessible Penn Dissertations. 1131. https://repository.upenn.edu/edissertations/1131 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/1131 For more information, please contact [email protected]. Ef-G:trna Dynamics During the Elongation Cycle of Protein Synthesis Abstract During polypeptide elongation cycle, prokaryotic elongation factor G (EF-G) catalyzes the coupled translocations on the ribosome of mRNA and A- and P-site bound tRNAs. Continued progress has been achieved in understanding this key process, including results of structural, ensemble kinetic and single- molecule studies. However, most of work has been focused on the pre-equilibrium states of this fast process, leaving the real time dynamics, especially how EF-G interacts with the A-site tRNA in the pretranslocation complex, not fully elucidated. In this thesis, the kinetics of EF-G catalyzed translocation is investigated by both ensemble and single molecule fluorescence resonance energy transfer studies to further explore the underlying mechanism. In the ensemble work, EF-G mutants were designed and expressed successfully. The labeled EF-G mutants show good translocation activity in two different assays. In the smFRET work, by attachment of a fluorescent probe at position 693 on EF-G permits monitoring of FRET efficiencies to sites in both ribosomal protein L11 and A-site tRNA. -

Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase
cells Review Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase Jaroslav Kalous *, Denisa Jansová and Andrej Šušor Institute of Animal Physiology and Genetics, Academy of Sciences of the Czech Republic, Rumburska 89, 27721 Libechov, Czech Republic; [email protected] (D.J.); [email protected] (A.Š.) * Correspondence: [email protected] Received: 28 April 2020; Accepted: 24 June 2020; Published: 27 June 2020 Abstract: Cyclin dependent kinase 1 (CDK1) has been primarily identified as a key cell cycle regulator in both mitosis and meiosis. Recently, an extramitotic function of CDK1 emerged when evidence was found that CDK1 is involved in many cellular events that are essential for cell proliferation and survival. In this review we summarize the involvement of CDK1 in the initiation and elongation steps of protein synthesis in the cell. During its activation, CDK1 influences the initiation of protein synthesis, promotes the activity of specific translational initiation factors and affects the functioning of a subset of elongation factors. Our review provides insights into gene expression regulation during the transcriptionally silent M-phase and describes quantitative and qualitative translational changes based on the extramitotic role of the cell cycle master regulator CDK1 to optimize temporal synthesis of proteins to sustain the division-related processes: mitosis and cytokinesis. Keywords: CDK1; 4E-BP1; mTOR; mRNA; translation; M-phase 1. Introduction 1.1. Cyclin Dependent Kinase 1 (CDK1) Is a Subunit of the M Phase-Promoting Factor (MPF) CDK1, a serine/threonine kinase, is a catalytic subunit of the M phase-promoting factor (MPF) complex which is essential for cell cycle control during the G1-S and G2-M phase transitions of eukaryotic cells. -

Disease-Related Cellular Protein Networks Differentially Affected
www.nature.com/scientificreports OPEN Disease‑related cellular protein networks diferentially afected under diferent EGFR mutations in lung adenocarcinoma Toshihide Nishimura1,8*, Haruhiko Nakamura1,2,8, Ayako Yachie3,8, Takeshi Hase3,8, Kiyonaga Fujii1,8, Hirotaka Koizumi4, Saeko Naruki4, Masayuki Takagi4, Yukiko Matsuoka3, Naoki Furuya5, Harubumi Kato6,7 & Hisashi Saji2 It is unclear how epidermal growth factor receptor EGFR major driver mutations (L858R or Ex19del) afect downstream molecular networks and pathways. This study aimed to provide information on the infuences of these mutations. The study assessed 36 protein expression profles of lung adenocarcinoma (Ex19del, nine; L858R, nine; no Ex19del/L858R, 18). Weighted gene co-expression network analysis together with analysis of variance-based screening identifed 13 co-expressed modules and their eigen proteins. Pathway enrichment analysis for the Ex19del mutation demonstrated involvement of SUMOylation, epithelial and mesenchymal transition, ERK/mitogen- activated protein kinase signalling via phosphorylation and Hippo signalling. Additionally, analysis for the L858R mutation identifed various pathways related to cancer cell survival and death. With regard to the Ex19del mutation, ROCK, RPS6KA1, ARF1, IL2RA and several ErbB pathways were upregulated, whereas AURK and GSKIP were downregulated. With regard to the L858R mutation, RB1, TSC22D3 and DOCK1 were downregulated, whereas various networks, including VEGFA, were moderately upregulated. In all mutation types, CD80/CD86 (B7), MHC, CIITA and IFGN were activated, whereas CD37 and SAFB were inhibited. Costimulatory immune-checkpoint pathways by B7/CD28 were mainly activated, whereas those by PD-1/PD-L1 were inhibited. Our fndings may help identify potential therapeutic targets and develop therapeutic strategies to improve patient outcomes. -
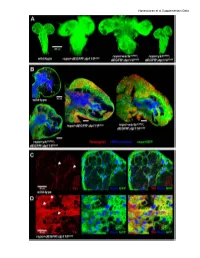
Supplementary Data Vigneswaran Et Al Supplementary Data
Vigneswaran et al Supplementary Data Vigneswaran et al Supplementary Data Figure S1: Yki is required for EGFR-PI3K-driven glial neoplasia in Drosophila (A) Optical projections of whole brain-nerve cord complexes from 3rd instar larvae approximately 130 hrs old. Dorsal view; anterior up. CD8-GFP (green) labels glial cell bodies. Compared to repo>dEGFRλ;dp110CAAX, warts knockdown (repo>wartsdsRNA; dEGFRλ;dp110CAAX) increased neoplastic brain overgrowth and yki knockdown (repo>ykidsRNA;dEGFRλ;dp110CAAX) decreased neoplastic brain overgrowth. (B) 3 µm optical projections of brain hemispheres, age-matched 3rd instar larvae. Frontal sections; anterior up; midline to left. Repo (red) labels glial cell nuclei; CD8-GFP (green) labels glial cell bodies; anti-HRP (blue) counter-stains for neurons and neuropil. (middle) repo>dEGFRλ;dp110CAAX showed increased glial cell numbers (red nuclei) compared to (upper left) wild-type. Compared to repo>dEGFRλ;dp110CAAX, (right) warts knockdown increased neoplastic glial cell numbers (red nuclei), whereas (lower left) yki knockdown reduced neoplastic glial cell numbers (red nuclei). (C, D) Low levels of Yki protein (red) was observed in wild-type central brain glia (white arrows, left panel in C) compared to high levels of cytoplasmic and nuclear Yki protein in dEGFRλ;dp110CAAX neoplastic glia (white arrows, left panel in D); Repo (blue) labels glial cell nuclei; CD8-GFP (green) labels glial cell bodies. Vigneswaran et al Supplementary Data Figure S2: YAP/TAZ expression confined to RTK-amplified tuMor cells and Maintained in patient-derived xenografts (A) On the left, immunohistochemical (IHC) staining in representative normal brain parenchyma in the cortex where YAP expression and TAZ expression was limited to vascular cells and was not detectable in normal neuronal and glial cells. -

Gene Section Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL INIST-CNRS Gene Section Review EEF1G (Eukaryotic translation elongation factor 1 gamma) Luigi Cristiano Aesthetic and medical biotechnologies research unit, Prestige, Terranuova Bracciolini, Italy; [email protected] Published in Atlas Database: March 2019 Online updated version : http://AtlasGeneticsOncology.org/Genes/EEF1GID54272ch11q12.html Printable original version : http://documents.irevues.inist.fr/bitstream/handle/2042/70656/03-2019-EEF1GID54272ch11q12.pdf DOI: 10.4267/2042/70656 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2020 Atlas of Genetics and Cytogenetics in Oncology and Haematology Abstract Keywords EEF1G; Eukaryotic translation elongation factor 1 Eukaryotic translation elongation factor 1 gamma, gamma; Translation; Translation elongation factor; alias eEF1G, is a protein that plays a main function protein synthesis; cancer; oncogene; cancer marker in the elongation step of translation process but also covers numerous moonlighting roles. Considering its Identity importance in the cell it is found frequently Other names: EF1G, GIG35, PRO1608, EEF1γ, overexpressed in human cancer cells and thus this EEF1Bγ review wants to collect the state of the art about EEF1G, with insights on DNA, RNA, protein HGNC (Hugo): EEF1G encoded and the diseases where it is implicated. Location: 11q12.3 Figure. 1. Splice variants of EEF1G. The figure shows the locus on chromosome 11 of the EEF1G gene and its splicing variants (grey/blue box). The primary transcript is EEF1G-001 mRNA (green/red box), but also EEF1G-201 variant is able to codify for a protein (reworked from https://www.ncbi.nlm.nih.gov/gene/1937; http://grch37.ensembl.org; www.genecards.org) Atlas Genet Cytogenet Oncol Haematol.