Latitudinal and Bathymetric Patterns in the Distribution and Abundance of Mesopelagic fish in the Scotia Sea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Review: the Energetic Value of Zooplankton and Nekton Species of the Southern Ocean
Marine Biology (2018) 165:129 https://doi.org/10.1007/s00227-018-3386-z REVIEW, CONCEPT, AND SYNTHESIS Review: the energetic value of zooplankton and nekton species of the Southern Ocean Fokje L. Schaafsma1 · Yves Cherel2 · Hauke Flores3 · Jan Andries van Franeker1 · Mary‑Anne Lea4 · Ben Raymond5,4,6 · Anton P. van de Putte7 Received: 8 March 2018 / Accepted: 5 July 2018 / Published online: 18 July 2018 © The Author(s) 2018 Abstract Understanding the energy fux through food webs is important for estimating the capacity of marine ecosystems to support stocks of living resources. The energy density of species involved in trophic energy transfer has been measured in a large number of small studies, scattered over a 40-year publication record. Here, we reviewed energy density records of Southern Ocean zooplankton, nekton and several benthic taxa, including previously unpublished data. Comparing measured taxa, energy densities were highest in myctophid fshes (ranging from 17.1 to 39.3 kJ g−1 DW), intermediate in crustaceans (7.1 to 25.3 kJ g−1 DW), squid (16.2 to 24.0 kJ g−1 DW) and other fsh families (14.8 to 29.9 kJ g−1 DW), and lowest in jelly fsh (10.8 to 18.0 kJ g−1 DW), polychaetes (9.2 to 14.2 kJ g−1 DW) and chaetognaths (5.0–11.7 kJ g−1 DW). Data reveals diferences in energy density within and between species related to size, age and other life cycle parameters. Important taxa in Antarctic food webs, such as copepods, squid and small euphausiids, remain under-sampled. -

Age and Growth of Brauer's Lanternfish Gymnoscopelus Braueri and Rhombic Lanternfish Krefftichthys Anderssoni (Family Myctophidae) in the Scotia Sea, Southern Ocean
Received: 22 July 2019 Accepted: 14 November 2019 DOI: 10.1111/jfb.14206 REGULAR PAPER FISH Age and growth of Brauer's lanternfish Gymnoscopelus braueri and rhombic lanternfish Krefftichthys anderssoni (Family Myctophidae) in the Scotia Sea, Southern Ocean Ryan A. Saunders1 | Silvia Lourenço2,3 | Rui P. Vieira4 | Martin A. Collins1 | Carlos A. Assis5,6 | Jose C. Xavier7,1 1British Antarctic Survey, Natural Environment Research Council, Cambridge, UK Abstract 2MARE - Marine and Environmental Sciences This study examines age and growth of Brauer's lanternfish Gymnoscopelus braueri and Centre, Polytechnic of Leiria, Peniche, Portugal rhombic lanternfish Krefftichthys anderssoni from the Scotia Sea in the Southern Ocean, 3CIIMAR/CIMAR, Interdisciplinary Centre of Marine and Environmental Research, through the analysis of annual growth increments deposited on sagittal otoliths. Oto- University of Porto, Matosinhos, Portugal lith pairs from 177 G. braueri and 118 K. anderssoni were collected in different seasons 4 Centre for Environment Fisheries and from the region between 2004 and 2009. Otolith-edge analysis suggested a seasonal Aquaculture Sciences, Lowestoft, UK change in opaque and hyaline depositions, indicative of an annual growth pattern, 5MARE-ULisboa – Marine and Environmental Sciences Centre, Faculty of Sciences, although variation within the populations of both species was apparent. Age estimates University of Lisbon, Lisbon, Portugal varied from 1 to 6 years for G. braueri (40 to 139 mm standard length; LS)andfrom 6Department of Animal Biology, Faculty of Sciences, University of Lisbon, Lisbon, Portugal 0 to 2 years for K. anderssoni (26 to 70 mm LS). Length-at-age data were broadly con- 7Department of Life Sciences, MARE-UC, sistent with population cohort parameters identified in concurrent length-frequency University of Coimbra, Coimbra, Portugal data from the region for both species. -

Order MYCTOPHIFORMES NEOSCOPELIDAE Horizontal Rows
click for previous page 942 Bony Fishes Order MYCTOPHIFORMES NEOSCOPELIDAE Neoscopelids By K.E. Hartel, Harvard University, Massachusetts, USA and J.E. Craddock, Woods Hole Oceanographic Institution, Massachusetts, USA iagnostic characters: Small fishes, usually 15 to 30 cm as adults. Body elongate with no photophores D(Scopelengys) or with 3 rows of large photophores when viewed from below (Neoscopelus).Eyes variable, small to large. Mouth large, extending to or beyond vertical from posterior margin of eye; tongue with photophores around margin in Neoscopelus. Gill rakers 9 to 16. Dorsal fin single, its origin above or slightly in front of pelvic fin, well in front of anal fins; 11 to 13 soft rays. Dorsal adipose fin over end of anal fin. Anal-fin origin well behind dorsal-fin base, anal fin with 10 to 14 soft rays. Pectoral fins long, reaching to about anus, anal fin with 15 to 19 rays.Pelvic fins large, usually reaching to anus.Scales large, cycloid, and de- ciduous. Colour: reddish silvery in Neoscopelus; blackish in Scopelengys. dorsal adipose fin anal-fin origin well behind dorsal-fin base Habitat, biology, and fisheries: Large adults of Neoscopelus usually benthopelagic below 1 000 m, but subadults mostly in midwater between 500 and 1 000 m in tropical and subtropical areas. Scopelengys meso- to bathypelagic. No known fisheries. Remarks: Three genera and 5 species with Solivomer not known from the Atlantic. All Atlantic species probably circumglobal . Similar families in occurring in area Myctophidae: photophores arranged in groups not in straight horizontal rows (except Taaningichthys paurolychnus which lacks photophores). Anal-fin origin under posterior dorsal-fin anal-fin base. -

Biodiversity of Arctic Marine Fishes: Taxonomy and Zoogeography
Mar Biodiv DOI 10.1007/s12526-010-0070-z ARCTIC OCEAN DIVERSITY SYNTHESIS Biodiversity of arctic marine fishes: taxonomy and zoogeography Catherine W. Mecklenburg & Peter Rask Møller & Dirk Steinke Received: 3 June 2010 /Revised: 23 September 2010 /Accepted: 1 November 2010 # Senckenberg, Gesellschaft für Naturforschung and Springer 2010 Abstract Taxonomic and distributional information on each Six families in Cottoidei with 72 species and five in fish species found in arctic marine waters is reviewed, and a Zoarcoidei with 55 species account for more than half list of families and species with commentary on distributional (52.5%) the species. This study produced CO1 sequences for records is presented. The list incorporates results from 106 of the 242 species. Sequence variability in the barcode examination of museum collections of arctic marine fishes region permits discrimination of all species. The average dating back to the 1830s. It also incorporates results from sequence variation within species was 0.3% (range 0–3.5%), DNA barcoding, used to complement morphological charac- while the average genetic distance between congeners was ters in evaluating problematic taxa and to assist in identifica- 4.7% (range 3.7–13.3%). The CO1 sequences support tion of specimens collected in recent expeditions. Barcoding taxonomic separation of some species, such as Osmerus results are depicted in a neighbor-joining tree of 880 CO1 dentex and O. mordax and Liparis bathyarcticus and L. (cytochrome c oxidase 1 gene) sequences distributed among gibbus; and synonymy of others, like Myoxocephalus 165 species from the arctic region and adjacent waters, and verrucosus in M. scorpius and Gymnelus knipowitschi in discussed in the family reviews. -

Guide to the Coastal Marine Fishes of California
STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 157 GUIDE TO THE COASTAL MARINE FISHES OF CALIFORNIA by DANIEL J. MILLER and ROBERT N. LEA Marine Resources Region 1972 ABSTRACT This is a comprehensive identification guide encompassing all shallow marine fishes within California waters. Geographic range limits, maximum size, depth range, a brief color description, and some meristic counts including, if available: fin ray counts, lateral line pores, lateral line scales, gill rakers, and vertebrae are given. Body proportions and shapes are used in the keys and a state- ment concerning the rarity or commonness in California is given for each species. In all, 554 species are described. Three of these have not been re- corded or confirmed as occurring in California waters but are included since they are apt to appear. The remainder have been recorded as occurring in an area between the Mexican and Oregon borders and offshore to at least 50 miles. Five of California species as yet have not been named or described, and ichthyologists studying these new forms have given information on identification to enable inclusion here. A dichotomous key to 144 families includes an outline figure of a repre- sentative for all but two families. Keys are presented for all larger families, and diagnostic features are pointed out on most of the figures. Illustrations are presented for all but eight species. Of the 554 species, 439 are found primarily in depths less than 400 ft., 48 are meso- or bathypelagic species, and 67 are deepwater bottom dwelling forms rarely taken in less than 400 ft. -
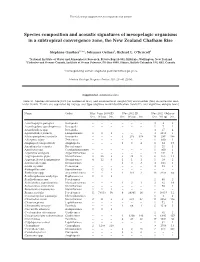
Marine Ecology Progress Series 503:23
The following supplement accompanies the article 1 Species composition and acoustic signatures of mesopelagic organisms in a subtropical convergence zone, the New Zealand Chatham Rise Stéphane Gauthier1, 2,*, Johannes Oeffner1, Richard L. O’Driscoll1 1National Institute of Water and Atmospheric Research, Private Bag 14-901, Kilbirnie, Wellington, New Zealand 2Fisheries and Oceans Canada, Institute of Ocean Sciences, PO Box 6000, Sidney, British Columbia V8L 4B2, Canada *Corresponding author: [email protected] Marine Ecology Progress Series: 503: 23–40 (2014) Supplement. Additional data Table S1. Species occurrence (Occ.) in number of tows, and abundance in weight (Wt) and number (No.) in successful mid - water trawls. Trawls are separated by voyage and type (daytime mark identification trawls ID, and nighttime oblique tows) Name Order May−June 2008 ID Nov 2011 ID Nov 2011 Oblique Occ. Wt (g) No. Occ. Wt (g) No. Occ. Wt (g) No. Acanthephyra pelagica Decapoda − − − − − − 3 9 3 Acanthephyra quadrispinosa Decapoda − − − − − − 1 7 1 Acanthephyra spp. Decapoda − − − − − − 3 17 4 Agrostichthys parkeri Lampriformes 1 8 1 − − − 3 1813 3 Alainopasiphaea australis Decapoda − − − 3 273 356 14 287 366 Allocyttus niger Zeiformes − − − − − − 1 600 1 Amphipod (unspecified) Amphipoda − − − 1 3 4 8 34 17 Anoplogaster cornuta Beryciformes − − − − − − 1 25 1 Apristurus spp. Carcharhiniformes − − − − − − 1 900 1 Argentina elongata Argentiniformes − − − − − − 4 541 6 Argyropelecus gigas Stomiiformes 2 20 2 2 38 5 8 165 12 Argyropelecus hemigymnus Stomiiformes 4 12 6 1 1 1 1 19 2 Astronesthes spp. Stomiiformes − − − 1 3 3 3 103 3 Atolla wyvillei Coronatae − − − − − − 4 83 7 Bathophilus ater Stomiiformes 1 12 1 − − − − − − Bathylagus spp. -

Biogeographic Atlas of the Southern Ocean
Census of Antarctic Marine Life SCAR-Marine Biodiversity Information Network BIOGEOGRAPHIC ATLAS OF THE SOUTHERN OCEAN CHAPTER 7. BIOGEOGRAPHIC PATTERNS OF FISH. Duhamel G., Hulley P.-A, Causse R., Koubbi P., Vacchi M., Pruvost P., Vigetta S., Irisson J.-O., Mormède S., Belchier M., Dettai A., Detrich H.W., Gutt J., Jones C.D., Kock K.-H., Lopez Abellan L.J., Van de Putte A.P., 2014. In: De Broyer C., Koubbi P., Griffiths H.J., Raymond B., Udekem d’Acoz C. d’, et al. (eds.). Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp. 328-362. EDITED BY: Claude DE BROYER & Philippe KOUBBI (chief editors) with Huw GRIFFITHS, Ben RAYMOND, Cédric d’UDEKEM d’ACOZ, Anton VAN DE PUTTE, Bruno DANIS, Bruno DAVID, Susie GRANT, Julian GUTT, Christoph HELD, Graham HOSIE, Falk HUETTMANN, Alexandra POST & Yan ROPERT-COUDERT SCIENTIFIC COMMITTEE ON ANTARCTIC RESEARCH THE BIOGEOGRAPHIC ATLAS OF THE SOUTHERN OCEAN The “Biogeographic Atlas of the Southern Ocean” is a legacy of the International Polar Year 2007-2009 (www.ipy.org) and of the Census of Marine Life 2000-2010 (www.coml.org), contributed by the Census of Antarctic Marine Life (www.caml.aq) and the SCAR Marine Biodiversity Information Network (www.scarmarbin.be; www.biodiversity.aq). The “Biogeographic Atlas” is a contribution to the SCAR programmes Ant-ECO (State of the Antarctic Ecosystem) and AnT-ERA (Antarctic Thresholds- Ecosys- tem Resilience and Adaptation) (www.scar.org/science-themes/ecosystems). Edited by: Claude De Broyer (Royal Belgian Institute -

Report of the Fourteenth Meeting of the Scientific Committee
SC-CAMLR-XIV SCIENTIFIC COMMITTEE FOR THE CONSERVATION OF ANTARCTIC MARINE LIVING RESOURCES REPORT OF THE FOURTEENTH MEETING OF THE SCIENTIFIC COMMITTEE HOBART, AUSTRALIA 23 - 27 OCTOBER, 1995 CCAMLR 25 Old Wharf Hobart Tasmania 7000 AUSTRALIA ______________________ Telephone: 61 02 310366 Facsimile: 61 02 232714 Chairman of the Scientific Committee Telex: AA 57236 November 1995 E-mail: [email protected] _____________________________________________________________________________________ This document is produced in the official languages of the Commission: English, French, Russian and Spanish. Copies are available from the CCAMLR Secretariat at the above address. 1 Abstract This document presents the adopted record of the Fourteenth Meeting of the Scientific Committee for the Conservation of Antarctic Marine Living Resources held in Hobart, Australia, from 23 to 27 October 1995. Major topics discussed at this meeting include: the CCAMLR ecosystem monitoring program, krill, fish, crab and squid resources, marine mammal and bird populations, assessment of incidental mortality, ecosystem monitoring and management, and management under conditions of uncertainty. Reports of meetings and intersessional activities of subsidiary bodies of the Scientific Committee, including the Working Groups on Ecosystem Monitoring and Management and on Fish Stock Assessment, are appended. TABLE OF CONTENTS OPENING OF THE MEETING Adoption of the Agenda Report of the Chairman FISHERY STATUS AND TRENDS Krill Fish Crabs Squid DEPENDENT SPECIES Species Monitored -

Role of Larval Stages in Systematic Investigations of Marine Teleosts: the Myctophidae, a Case Study
ROLE OF LARVAL STAGES IN SYSTEMATIC INVESTIGATIONS OF MARINE TELEOSTS: THE MYCTOPHIDAE, A CASE STUDY H. GEOFFREYMOSER AND ELBERTH. AHUXTROM~ ABSTRACT The lanternfish family Myctophidae is the most speciose and widespread family of mid-water fishes in the world man. As presently recognized it contains about 30 genera and 300 nominal species. Their larvae are highly prominent in the plankton and make up about 509b of all larvae taken in open-oeean plankton tows. Our studies of myctophid larvae, on a worldwide basis, have demonstrated that characters of the larval stages of lanternfishes are of great utility in systematic analysis. The genera and species can be recognized on the basis of eye and body shape, the shape and length of the gut, and pigment pattern and by the sequence of photophore development. In this paper the larvae of 55 species representing 24 genera are illustrated and used to demonstrate the usefulness of larvae in understanding the relation- ships of species within genera. Characters of the larvae provide insight into generic anities of lanternfish, allowing us to construct an evolutionary scheme of tribes and subfamilies that differs in some aspects from those proposed on the basis of adult characters. The concept of using larval characters in combination with adult characters to delineate phylogenetic lines in myctophids is discussed, as is our view of evolutionary strategy in the family. A major facet of comprehensive systematic inves- played a large role in the taxonomy of anguil- tigations is the search for functionally unrelated liform fishes (Castle, 1969) partly because of the characters. Whether the independence of these conspicuousnessof eel leptocephali and partly be- characters is actual or merely apparent, they con- cause of the unavailability of adults of many of the stitute useful elements in the analysis of systema- families. -

Diet of Nineteen Mesopelagic Fishes in the Gulf of Alaska
NOAA Technical Memorandum NMFS-AFSC-229 Diet of Nineteen Mesopelagic Fishes in the Gulf of Alaska by M-S. Yang U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center November 2011 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Yang, M-S. 2011. Diet of nineteen mesopelagic fishes in the Gulf of Alaska. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-229, 67 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-229 Diet of Nineteen Mesopelagic Fishes in the Gulf of Alaska by M-S. Yang Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle, WA 98115 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE John E. Bryson, Secretary National Oceanic and Atmospheric Administration Jane Lubchenco, Under Secretary and Administrator National Marine Fisheries Service Eric C. Schwaab, Assistant Administrator for Fisheries November 2011 This document is available to the public through: National Technical Information Service U.S.