A Reference Guide Maryland Herpetofauna Taxonomy Tips
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nonnative Reptilies in South Florida ID Guide
Nonnative Reptiles in South Florida Identification Guide • The nonnative reptiles shown here are native to Central and South America, Asia, and Nonnative species are Africa. They were introduced to south Florida by human activity. sometimes confused with • Invasive species harm native species through direct predation, competition for resources, the Florida natives shown spread of disease, and disruption of natural ecosystems. Many of the nonnative reptiles on because their colorations this guide are, or have the potential to become, invasive. and patterns are very • Use this guide to identify invasive species and immediately report sightings of the black similar. Pay attention to the and white tegu, Nile monitor, and all invasive snakes to 1-888-IVE-GOT1. Take a distinct characteristics and photo and note the location relative to street intersections or with a GPS if possible. typical adult sizes listed on this guide to avoid • More photos can be found at www.flmnh.ufl.edu/herpetology/herpetology.htm. confusion when you • Be certain that an animal is a nonnative species before removing it. Warning-most encounter these animals. reptiles will bite or scratch if provoked. Nonnative Lizards NATIVE :- • ,,.., •· t ..... Look-a-Likes . ... ·-tt-..... • •. .. l . 1 '\..\ =- ' . ----.....·~·-· - - ',-<•'-' ' . \:,' . <! •.t'- . ,. '\. Dav id 13,irbsv ~ ·- ~ 9111'.', o:'"' w:' Black and White Tegu 2 to 3 ft. Dark bands with plentiful white dots between them Eastern Fence Lizard 3.5 to 7.5 in. Northern Curly-Tailed Lizard 7 to 10.5 in . Gray to tan with curled tail Florida Scrub Lizard 3.5 to 5.5 in. American Alligator 6 to 9 ft. Nile Monitor 4 to 6 ft. -

Indiana Snakes Are Listed Here, and Not All Streams, Ponds and Lakes Suns Beside Creeks
Midwest Worm Snake Carphophis amoenus satiny gray below with a brown or dark amber Fox Snake Elaphe vulpina This snake version of the earthworm is iris of the eye. The blue racer may show Blue This snake of marshes and wet places has brown above and has a pink belly and sides. It varying shades of gunmetal gray or blue above bold blotches, a grayish- or brownish-yellow is secretive and seldom seen, spending most of and below with a darker head and eye area. Mixed body and a dull orange/reddish head and tail. its time under stones, boards and logs where Racers move fast and sometimes appear to Black It vibrates its tail if cornered, but rarely bites. the ground is moist. It feeds on soft-bodied “chase” people. In fact, this behavior is often Snakes insects and earthworms. associated with courtship and may be used to drive an Black Kingsnake Lampropeltis getulus getulus intruder out of a territory. This glossy black snake has speckles of Rough Green Snake Opheodrys aestivas white and cream that may be less apparent in Smooth Green Snake Opheodrys vernalis Eastern Milk Snake older snakes. It lives on streambanks and in Both species are green above with white, Lampropeltis triangulatum triangulatum moist meadows, where it feeds on other yellow or pale green bellies. The rough green Red Milk Snake snakes, turtle eggs, mice and voles. It is snake has keeled scales that give it a rough Lampropeltis triangulatum syspila “V” generally secretive and can be found under texture. This snake, listed as a species of This snake’s taste for mice makes pattern boards, logs and debris. -

A Herpetofaunal Survey of the Santee National Wildlife Refuge Submitted
A Herpetofaunal Survey of the Santee National Wildlife Refuge Submitted to the U.S. Fish and Wildlife Service October 5, 2012 Prepared by: Stephen H. Bennett Wade Kalinowsky South Carolina Department of Natural Resources Introduction The lack of baseline inventory data of herpetofauna on the Santee National Wildlife Refuge, in general and the Dingle Pond Unit specifically has proven problematic in trying to assess priority species of concern and direct overall management needs in this system. Dingle Pond is a Carolina Bay which potentially provides unique habitat for many priority reptiles and amphibians including the federally threatened flatwoods salamander, the state endangered gopher frog, state threatened dwarf siren and spotted turtle and several species of conservation concern including the tiger salamander, upland chorus frog (coastal plain populations only), northern cricket frog (coastal plain populations only), many-lined salamander, glossy crayfish snake and black swamp snake. The presence or abundance of these and other priority species in this large Carolina Bay is not known. This project will provide for funds for South Carolina DNR to conduct baseline surveys to census and assess the status of the herpetofauna in and adjacent to the Dingle Pond Carolina Bay. Surveys will involve a variety of sampling techniques including funnel traps, hoop traps, cover boards, netting and call count surveys to identify herpetofauna diversity and abundance. Herpetofauna are particularly vulnerable to habitat changes including climate change and human development activities. Many unique species are endemic to Carolina Bays, a priority habitat that has been greatly diminished across the coastal plain of South Carolina. These species can serve as indicator species of habitat quality and climate changes and baseline data is critical at both the local and regional level. -

Appendices to the Final Environmental Impact Statement
Daniel Boone National Forest Appendix H Appendix H VIABILITY EVALUATION TABLES TERRESTRIAL HABITAT ELEMENTS, TABLE H – 1 Outcomes for terrestrial habitat elements are provided in Table H - 1, using the four variables described in the Terrestrial Species Viability Evaluation section of Chapter 3. These variables indicate expected habitat condition following 50 years of implementing each alternative. Key to Table H – 1 Habitat Abundance – Values used to categorize projected abundance of each habitat element after 50 years of implementing each forest plan revision alternative. Code Description R Rare. The habitat element is rare, with generally less than 100 occurrences, or patches of the element generally covering less than one percent of the planning area. O Occasional. The habitat element is encountered occasionally, and generally found on one to ten percent of the planning area. C Common. The habitat element is abundant and frequently encountered, and generally found on more than ten percent of the planning area. Habitat Distribution – Values used to categorize projected distribution of each habitat element after 50 years of implementing each forest plan revision alternative. Code Description P Poor. The habitat element is poorly distributed within the planning area and intermixed lands relative to conditions present prior to European settlement. Number and size of high quality habitat patches is greatly reduced. F Fair. The habitat element is fairly well distributed within the planning area and intermixed lands relative to conditions present prior to European settlement. Number and size of high quality habitat patches is somewhat reduced. G Good. The habitat element is well distributed within the planning area and intermixed lands relative to conditions present prior to European settlement. -
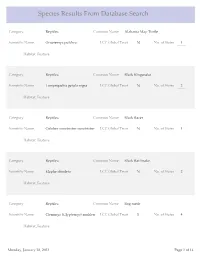
Species Results from Database Search
Species Results From Database Search Category Reptiles Common Name Alabama Map Turtle Scientific Name Graptemys pulchra LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Kingsnake Scientific Name Lampropeltis getula nigra LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Black Racer Scientific Name Coluber constrictor constrictor LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Rat Snake Scientific Name Elaphe obsoleta LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Bog turtle Scientific Name Clemmys (Glyptemys) muhlen LCC Global Trust Y No. of States 4 Habitat_Feature Monday, January 28, 2013 Page 1 of 14 Category Reptiles Common Name Broadhead Skink Scientific Name Eumeces laticeps LCC Global Trust N No. of States 5 Habitat_Feature Category Reptiles Common Name Coal Skink Scientific Name Eumeces anthracinus LCC Global Trust Y No. of States 8 Habitat_Feature Category Reptiles Common Name Common Five-lined Skink Scientific Name Eumeces fasciatus LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Common Map Turtle Scientific Name Graptemys geographica LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Musk Turtle Scientific Name Sternotherus odoratus LCC Global Trust N No. of States 2 Habitat_Feature Monday, January 28, 2013 Page 2 of 14 Category Reptiles Common Name Common Ribbonsnake Scientific Name Thamnophis sauritus sauritus LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Snapping Turtle Scientific Name Chelydra serpentina LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Corn snake Scientific Name Elaphe guttata guttata LCC Global Trust N No. -

Wildlife Habitat Plan
WILDLIFE HABITAT PLAN City of Novi, Michigan A QUALITY OF LIFE FOR THE 21ST CENTURY WILDLIFE HABITAT PLAN City of Novi, Michigan A QUALIlY OF LIFE FOR THE 21ST CENTURY JUNE 1993 Prepared By: Wildlife Management Services Brandon M. Rogers and Associates, P.C. JCK & Associates, Inc. ii ACKNOWLEDGEMENTS City Council Matthew C. Ouinn, Mayor Hugh C. Crawford, Mayor ProTem Nancy C. Cassis Carol A. Mason Tim Pope Robert D. Schmid Joseph G. Toth Planning Commission Kathleen S. McLallen, * Chairman John P. Balagna, Vice Chairman lodia Richards, Secretary Richard J. Clark Glen Bonaventura Laura J. lorenzo* Robert Mitzel* Timothy Gilberg Robert Taub City Manager Edward F. Kriewall Director of Planning and Community Development James R. Wahl Planning Consultant Team Wildlife Management Services - 640 Starkweather Plymouth, MI. 48170 Kevin Clark, Urban Wildlife Specialist Adrienne Kral, Wildlife Biologist Ashley long, Field Research Assistant Brandon M. Rogers and Associates, P.C. - 20490 Harper Ave. Harper Woods, MI. 48225 Unda C. lemke, RlA, ASLA JCK & Associates, Inc. - 45650 Grand River Ave. Novi, MI. 48374 Susan Tepatti, Water Resources Specialist * Participated with the Planning Consultant Team in developing the study. iii TABLE OF CONTENTS ACKNOWLEDGEMENTS iii PREFACE vii EXECUTIVE SUMMARY viii FRAGMENTATION OF NATURAL RESOURCES " ., , 1 Consequences ............................................ .. 1 Effects Of Forest Fragmentation 2 Edges 2 Reduction of habitat 2 SPECIES SAMPLING TECHNIQUES ................................ .. 3 Methodology 3 Survey Targets ............................................ ., 6 Ranking System ., , 7 Core Reserves . .. 7 Wildlife Movement Corridor .............................. .. 9 FIELD SURVEY RESULTS AND RECOMMENDATIONS , 9 Analysis Results ................................ .. 9 Core Reserves . .. 9 Findings and Recommendations , 9 WALLED LAKE CORE RESERVE - DETAILED STUDy.... .. .... .. .... .. 19 Results and Recommendations ............................... .. 21 GUIDELINES TO ECOLOGICAL LANDSCAPE PLANNING AND WILDLIFE CONSERVATION. -

Hybridization Between Multiple Fence Lizard Lineages in an Ecotone
Molecular Ecology (2007) 16, 1035–1054 doi: 10.1111/j.1365-294X.2006.03194.x HybridizationBlackwell Publishing Ltd between multiple fence lizard lineages in an ecotone: locally discordant variation in mitochondrial DNA, chromosomes, and morphology ADAM D. LEACHÉ* and CHARLES J. COLE†‡ *Museum of Vertebrate Zoology and Department of Integrative Biology, 3101 Valley Life Sciences Building, University of California, Berkeley, CA 94720-3160, USA, †Department of Herpetology, American Museum of Natural History, New York, NY 10024-5192, USA Abstract We investigated a hybrid zone between two major lineages of fence lizards (Sceloporus cowlesi and Sceloporus tristichus) in the Sceloporus undulatus species complex in eastern Arizona. This zone occurs in an ecotone between Great Basin Grassland and Conifer Wood- land habitats. We analysed spatial variation in mtDNA (N = 401; 969 bp), chromosomes (N = 217), and morphology (N = 312; 11 characters) to characterize the hybrid zone and assess species limits. A fine-scale population level phylogenetic analysis refined the boundaries between these species and indicated that four nonsister mtDNA clades (three belonging to S. tristichus and one to S. cowlesi) are sympatric at the centre of the zone. Esti- mates of cytonuclear disequilibria in the population closest to the centre of the hybrid zone suggest that the S. tristichus clades are randomly mating, but that the S. cowlesi haplotype has a significant nonrandom association with nuclear alleles. Maximum-likelihood cline- fitting analyses suggest that the karyotype, morphology, and dorsal colour pattern clines are all coincident, but the mtDNA cline is skewed significantly to the south. A temporal comparison of cline centres utilizing karyotype data collected in the early 1970s and in 2002 suggests that the cline may have shifted by approximately 1.5 km to the north over a 30-year period. -

Frogs and Toads of Rhode Island
FROG & TOAD Chug-o-rum! Peep-peeep-peeep! Kraack-arrack! Frogs and toads thrive in the many ponds, forests , felds and open woodlands found on Audubon wildlife refuges. Spring and summer are great times to look and listen for them. Listed below are the common species found in Rhode Island. Bullfrog Northern Leopard Frog (Rana catesbeiana) (Rana pipiens) Bullfrogs are the largest frog in the United States Leopard frogs, as their name implies, are covered and have smooth, dull green to brown skin with in many, round black spots with lighter outlines. dark spots. Males have a yellow chin, and females Teir base color is usually brown or green but can have a white chin. Males make a deep echoing vary into yellow or blue. Tey have raised, golden croak “Chug-o-rum, Chug-o-rum” call in early ridges running down their back. Tey can be found summer. It can sound like a cow mooing, which in meadows and grasslands in the warmer months is why the word “bull” is in its name. Tey eat and near ponds and streams during breeding season. many insects, but will also eat fsh, rodents and Teir calls sound like a mix of “chucks” and “old small birds. creaky door” sounds. Green Frog Pickerel Frog (Rana clamitans) (Rana palustris) Green frogs greatly resemble bullfrogs, with a bright Nearly the twin of the leopard frog, pickerel green head and shoulders and hints of brown, blue or frogs are distinguished by their spots being black, but can be distinguished by the raised ridges square shaped rather than round and a bright down their backs. -

The Case of the Missing Frogs by Bryan Hamilton Why Are There No
The Case of the Missing Frogs By Bryan Hamilton Why are there no frogs in Great Basin National Park? Great Basin National Park seems like a perfect refuge for frogs, with plenty of water--ten perennial streams, hundreds of springs, and six alpine lakes, but apparently there are no amphibians. During 2002, an amphibian inventory was conducted within Great Basin National Park. Crews surveyed perennial streams, springs, and alpine lakes, but did not observe any adult amphibians, tadpoles, or egg masses. The reasons for this are unclear, but apparently frog distribution depends on more than just abundant water. Lack of suitable breeding habitat is a major limiting factor in amphibian distribution. Most amphibians require still or extremely slow moving water to lay their eggs in. High gradient streams in the Park do not provide appropriate breeding habitat. The hundreds of springs in the Park may provide this habitat, however, no amphibians were found at the dozens of springs surveyed this year. Isolation from source populations is another limiting factor. Streams leave the Park and then quickly disappear underground. Amphibian populations near the Park are not directly connected to these streams. Thus no corridors exist between the Park and nearby amphibian populations. One amphibian species that does not require aquatic corridors for migration is the Great Basin Spadefoot (Spea intermontana), commonly referred to as a "toad." Spadefoots are distinguished from "true toads" (members of the family Bufonidae) by a black wedge shaped spade on their hind feet, used for burrowing. Spadefoots move considerable distances over land during spring and summer rains, travelling to breeding sites. -

Rhinella Marina) on Sanibel Island, FL
Possible Introduction Mechanisms, Movement Patterns, and Control Efforts of the Giant Toad (Rhinella marina) on Sanibel Island, FL Chris Lechowicz Director-Wildlife Habitat Management Program/Herpetologist Sanibel-Captiva Conservation Foundation Giant Toad (Rhinella marina) • Also called the cane toad, marine toad, Bufo toad, faux toad. • Previously Bufo marinus • Native to Central America, South America, Mexico, and south Texas. • A true toad (Family Bufonidae) belonging to a group of Neotropical toads (beaked toads = Rhinella). • Largest Bufonid toad (up to 5.8 lb), but not the largest Anuran (frog) in the world. Notable Facts • They have very large paired parotoid glands containing Bufotoxin that is oozed out when harassed. • This milky toxin has killed household pets (dogs, cats) and numerous wildlife species when ingested. • There has been documented human fatalities from “toad- licking” and ingestion. • Eggs/larvae are toxic to wildlife. • Highly nocturnal Prey and Predators • They consume invertebrates, small rodents, birds, amphibians, reptiles , and plants. • They will eat non-living prey (small dead animals, dog and food, feces). • The are preyed on in their natural range by caimans (C. latirostris), certain species of fish, Possums (Didelphis species), meat ants, banded cat-eyed snake (Leptodeira annulata), and some species of ibis. • They can lay up to ~30,000 eggs a year. Current uses for Cane Toads • Educational purposes (the dangers of exotic species) • Pregnancy tests • Leather goods • Pet trade • Eaten in Peru (after -

Species of Greatest Conservation Need Species Accounts
2 0 1 5 – 2 0 2 5 Species of Greatest Conservation Need Species Accounts Appendix 1.4C-Amphibians Amphibian Species of Greatest Conservation Need Maps: Physiographic Provinces and HUC Watersheds Species Accounts (Click species name below or bookmark to navigate to species account) AMPHIBIANS Eastern Hellbender Northern Ravine Salamander Mountain Chorus Frog Mudpuppy Eastern Mud Salamander Upland Chorus Frog Jefferson Salamander Eastern Spadefoot New Jersey Chorus Frog Blue-spotted Salamander Fowler’s Toad Western Chorus Frog Marbled Salamander Northern Cricket Frog Northern Leopard Frog Green Salamander Cope’s Gray Treefrog Southern Leopard Frog The following Physiographic Province and HUC Watershed maps are presented here for reference with conservation actions identified in the species accounts. Species account authors identified appropriate Physiographic Provinces or HUC Watershed (Level 4, 6, 8, 10, or statewide) for specific conservation actions to address identified threats. HUC watersheds used in this document were developed from the Watershed Boundary Dataset, a joint project of the U.S. Dept. of Agriculture-Natural Resources Conservation Service, the U.S. Geological Survey, and the Environmental Protection Agency. Physiographic Provinces Central Lowlands Appalachian Plateaus New England Ridge and Valley Piedmont Atlantic Coastal Plain Appalachian Plateaus Central Lowlands Piedmont Atlantic Coastal Plain New England Ridge and Valley 675| Appendix 1.4 Amphibians Lake Erie Pennsylvania HUC4 and HUC6 Watersheds Eastern Lake Erie -

AMPHIBIANS of OHIO F I E L D G U I D E DIVISION of WILDLIFE INTRODUCTION
AMPHIBIANS OF OHIO f i e l d g u i d e DIVISION OF WILDLIFE INTRODUCTION Amphibians are typically shy, secre- Unlike reptiles, their skin is not scaly. Amphibian eggs must remain moist if tive animals. While a few amphibians Nor do they have claws on their toes. they are to hatch. The eggs do not have are relatively large, most are small, deli- Most amphibians prefer to come out at shells but rather are covered with a jelly- cately attractive, and brightly colored. night. like substance. Amphibians lay eggs sin- That some of these more vulnerable spe- gly, in masses, or in strings in the water The young undergo what is known cies survive at all is cause for wonder. or in some other moist place. as metamorphosis. They pass through Nearly 200 million years ago, amphib- a larval, usually aquatic, stage before As with all Ohio wildlife, the only ians were the first creatures to emerge drastically changing form and becoming real threat to their continued existence from the seas to begin life on land. The adults. is habitat degradation and destruction. term amphibian comes from the Greek Only by conserving suitable habitat to- Ohio is fortunate in having many spe- amphi, which means dual, and bios, day will we enable future generations to cies of amphibians. Although generally meaning life. While it is true that many study and enjoy Ohio’s amphibians. inconspicuous most of the year, during amphibians live a double life — spend- the breeding season, especially follow- ing part of their lives in water and the ing a warm, early spring rain, amphib- rest on land — some never go into the ians appear in great numbers seemingly water and others never leave it.