Advances in Concentrating Solar Thermal Research and Technology Related Titles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Impact Assessment
Environmental Impact Assessment Study for the proposed Concentrated Solar Power Plant (Parabolic Trough) on the farm Sand Draai 391, Northern Cape – Environmental Scoping Report A Report for Solafrica 14/12/16/3/3/3/203 – Parabolic Trough DOCUMENT DESCRIPTION Client: Solafrica Energy (Pty) Ltd Project Name: Environmental Impact Assessment Study for the proposed Concentrated Solar Power Plant (Parabolic Trough) on the farm Sand Draai 391, Northern Cape Royal HaskoningDHV Reference Number: T01.JNB.000565 Authority Reference Number: 14/12/16/3/3/3/203 – Parabolic Trough Compiled by: Johan Blignaut Date: July 2015 Location: Woodmead Review: Prashika Reddy & Malcolm Roods Approval: Malcolm Roods _____________________________ Signature © Royal HaskoningDHV All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, without the written permission from Royal HaskoningDHV. Table of Contents 1 INTRODUCTION ........................................................................................................................................... 1 1.1 Background ............................................................................................................................................ 1 1.2 Need and Desirability ............................................................................................................................. 1 1.2.1 Renewable Energy Independent Power Producers Programme (REIPPPP) and Integrated Resource Plan (2010) .................................................................................................................... -

Analysis of New International Interconnectors to the South African Power System
Analysis of new international interconnectors to the South African power system 08-01-2016 1 2 Table of contents Key findings .......................................................................................................... 4 Introduction .......................................................................................................... 6 The South African power system ........................................................................... 7 Methodology and scenarios ................................................................................... 9 Scenarios .............................................................................................................. 11 Reference scenario ............................................................................................... 11 Hydro import scenarios ........................................................................................ 12 Value of interconnectors ...................................................................................... 13 Main results and conclusions ............................................................................... 15 Economic consequences for the system .............................................................. 17 Value of increasing interconnector capacity internally in South Africa ............... 19 Conclusion ............................................................................................................ 20 Detailed results of the scenario analysis .............................................................. -

CSP Technologies
CSP Technologies Solar Solar Power Generation Radiation fuel Concentrating the solar radiation in Concentrating Absorbing Storage Generation high magnification and using this thermal energy for power generation Absorbing/ fuel Reaction Features of Each Types of Solar Power PTC Type CRS Type Dish type 1Axis Sun tracking controller 2 Axis Sun tracking controller 2 Axis Sun tracking controller Concentrating rate : 30 ~ 100, ~400 oC Concentrating rate: 500 ~ 1,000, Concentrating rate: 1,000 ~ 10,000 ~1,500 oC Parabolic Trough Concentrator Parabolic Dish Concentrator Central Receiver System CSP Technologies PTC CRS Dish commercialized in large scale various types (from 1 to 20MW ) Stirling type in ~25kW size (more than 50MW ) developing the technology, partially completing the development technology development is already commercialized efficiency ~30% reached proper level, diffusion level efficiency ~16% efficiency ~12% CSP Test Facilities Worldwide Parabolic Trough Concentrator In 1994, the first research on high temperature solar technology started PTC technology for steam generation and solar detoxification Parabolic reflector and solar tracking system were developed <The First PTC System Installed in KIER(left) and Second PTC developed by KIER(right)> Dish Concentrator 1st Prototype: 15 circular mirror facets/ 2.2m focal length/ 11.7㎡ reflection area 2nd Prototype: 8.2m diameter/ 4.8m focal length/ 36㎡ reflection area <The First(left) and Second(right) KIER’s Prototype Dish Concentrator> Dish Concentrator Two demonstration projects for 10kW dish-stirling solar power system Increased reflection area(9m dia. 42㎡) and newly designed mirror facets Running with Solo V161 Stirling engine, 19.2% efficiency (solar to electricity) <KIER’s 10kW Dish-Stirling System in Jinhae City> Dish Concentrator 25 20 15 (%) 10 발전 효율 5 Peak. -

Responses to Budget & Finance Committee Questions on SEIP
Responses to Budget & Finance Committee Questions on the Pilot Solar Energy Incentive Program GoSolarSF Power Enterprise, SFPUC Introduction to the Program The San Francisco Public Utilities Commission proposed to initiate, as a pilot program, the Solar Energy Incentive Payment program, pending before Budget and Finance Committee. The pilot program would provide a financial incentive for San Francisco residents and businesses to install solar (photovoltaic) energy systems on their properties. The pilot Solar Energy Incentive Program, announced in December 2007, and supported by the Public Utilities Commission in Resolution 08-0004, would provide from $3,000 to $5,000 for residential installations and up to $10,000 for commercial photovoltaic installations. The pilot program would offer a one-time incentive payment for local solar electric projects to reduce the cost of installation. The pilot program would have three distinct incentives for residential installations. The incentives are limits on assistance, meaning that the higher incentives are not additive to the lower ones, but are total incentives. The incentive for businesses is a simple capacity-based incentive. Residents Businesses Basic Incentive $3,000 City installer incentive $4,000 $1.50 per Watt a system is designed to generate, up to a cap of $10,000. Environmental justice $5,000 incentive Incentive payments are tied to individual electric meters, meaning that buildings with more than one meter or applicants owning more than one property are eligible for more than one incentive payment, subject to the provisions below. Any system whose California Solar Initiative incentive reservation date is on or after December 11th 2007 would be eligible for the pilot San Francisco incentive. -
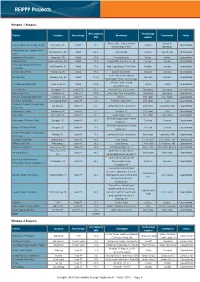
REIPPP Projects
REIPPP Projects Window 1 Projects Net capacity Technology Project Location Technology Developer Contractor Status MW supplier Klipheuwel – Dassiefontein Group 5, Dassiesklip Wind Energy Facility Caledon, WC Wind 26,2 Sinovel Operational Wind Energy fFcility Iberdrola MetroWind Van Stadens Wind Port Elizabeth, EC Wind 26,2 MetroWind Sinovel Basil Read Operational Farm Hopefield Wind Farm Hopefield, WC Wind 65,4 Umoya Energy Vestas Vestas Operational Noblesfontein Noblesfontein, NC Wind 72,8 Coria (PKF) Investments 28 Vestas Vestas Operational Red Cap Kouga Wind Farm – Port Elizabeth, EC Wind 77,6 Red Cap Kouga Wind Farm Nordex Nordex Operational Oyster Bay Dorper Wind Farm Stormberg, EC Wind 97,0 Dorper Wind Farm Nordex Nordex Operational South Africa Mainstream Jeffreys Bay Jeffereys Bay, EC Wind 133,9 Siemens Siemens Operational Renewable Power Jeffreys Bay African Clean Energy Cookhouse Wind Farm Cookhouse, EC Wind 135,0 Suzlon Suzlon Operational Developments Khi Solar One Upington, NC Solar CSP 50,0 Khi Dolar One Consortium Abengoa Abengoa Construction KaXu Solar One Pofadder, NC Solar CSP 100,0 KaXu Solar One Consortium Abengoa Abengoa Operational SlimSun Swartland Solar Park Swartland, WC Solar PV 5,0 SlimSun BYD Solar Juwi, Hatch Operational RustMo1 Solar Farm Rustenburg, NWP Solar PV 6,8 RustMo1 Solar Farm BYD Solar Juwi Operational Mulilo Renewable Energy Solar De Aar, NC Solar PV 9,7 Gestamp Mulilo Consortium Trina Solar Gestamp, ABB Operational PV De Aar Konkoonsies Solar Pofadder, NC Solar PV 9,7 Limarco 77 BYD Solar Juwi Operational -

Optimising the Concentrating Solar Power Potential in South Africa Through an Improved GIS Analysis
energies Article Optimising the Concentrating Solar Power Potential in South Africa through an Improved GIS Analysis Dries. Frank Duvenhage 1,* , Alan C. Brent 1,2 , William H.L. Stafford 1,3 and Dean Van Den Heever 4 1 Engineering Management and Sustainable Systems, Department of Industrial Engineering, the Solar Thermal Energy Research Group and the Centre for Renewable and Sustainable Energy Studies, Stellenbosch University, Stellenbosch 7602, South Africa; [email protected] (A.C.B.); wstaff[email protected] (W.H.L.S.) 2 Sustainable Energy Systems, School of Engineering and Computer Science, Victoria University of Wellington, Wellington 6140, New Zealand 3 Green Economy Solutions, Natural Resources and the Environment, Council for Scientific and Industrial Research, Stellenbosch 7600, South Africa 4 Legal Drone Solutions, Stellenbosch 7600, South Africa; [email protected] * Correspondence: [email protected] Received: 11 May 2020; Accepted: 16 June 2020; Published: 23 June 2020 Abstract: Renewable Energy Technologies are rapidly gaining uptake in South Africa, already having more than 3900 MW operational wind, solar PV, Concentrating Solar Power (CSP) and biogas capacity. CSP has the potential to become a leading Renewable Energy Technology, as it is the only one inherently equipped with the facility for large-scale thermal energy storage for increased dispatchability. There are many studies that aim to determine the potential for CSP development in certain regions or countries. South Africa has a high solar irradiation resource by global standards, but few studies have been carried out to determine the potential for CSP. One such study was conducted in 2009, prior to any CSP plants having been built in South Africa. -

The Case of Bokpoort CSP Plant in South Africa
Dispatchable Solar Energy 24/7 – The Case of Bokpoort CSP plant in South Africa Nandu Bhula Deputy Managing Director (Southern Africa) At A Glance We develop power and desalination water plants In over a decade we have become the second largest power & water developer in the GCC region, and a name to contend with internationally. We have achieved this by developing, investing in and operating a world-class 2004 portfolio... Saudi Arabia 58 37.7+ 5.8 Assets* GW Power* Mm3 per day Desalinated Water* 2008 – 2010 Oman 12 $59+ bn 21.5% Jordan Countries USD of Assets Portfolio in Renewable Under Energy based on Management** Share of project cost 2012 – 2014 3,500+ 30+ ~60% Morocco Employees Nationalities Local South Africa Employment Turkey in projects * Figures inclusive of advanced development projects ** There is another $10.5 bn of projects in advanced development DEVELOP INVEST OPERATE 2015 – UAE We win bids as lead developer, While taking significant, We operate and maintain our by partnering with the best and long-term stakes in all our plants plants to the highest global Egypt focusing on cost leadership. standards Vietnam 15/12/2020 CSP – Status across the Globe •CSP technology implemented in 23 countries 30 000 across the globe 25 000 20 000 •Over 6,000MW in operation 15 000 10 000 •Over 3,500MW in construction 5 000 - •Almost 25,000MW planned or in development Planned CSP Under Operational stages Projects Construction Source: https://solargis.com/maps-and-gis-data/overview/ 15/12/2020 ACWA Power CSP’s in MENA Region 150MW 950MW – (3 x 200MW CSP Trough, 1 x - Tower CSP+PV Hybrid 100MW CSP Tower & 250MW PV) 200MW - Trough 160MW - Trough 510MW – CSP Complex 15/12/2020 South African Renewable Energy IPP Program (REIPPP) • Started in 2011, the REIPPP is widely recognized as one of the most successful renewable energy procurement models in the world. -

Solar Thermal and Concentrated Solar Power Barometers 1 – EUROBSERV’ER –JUIN 2017 – EUROBSERV’ER BAROMETERS POWER SOLAR CONCENTRATED and THERMAL SOLAR
1 2 - 4.6% The decrease of the solar thermal market in the European Union in 2016 Evacuated tube solar collectors, solar thermal installation in Ireland SOLAR THERMAL AND CONCENTRATED SOLAR POWER BAROMETERS A study carried out by EurObserv’ER. solar solar concentrated and thermal power barometers solar solar concentrated and thermal power barometers he European solar thermal market is still losing pace. According to the Tpreliminary estimates from EurObserv’ER, the solar thermal segment dedicated to heat production (domestic hot water, heating and heating networks) contracted by a further 4.6% in 2016 down to 2.6 million m2. The sector is pinning its hopes on the development of the collective solar segment that includes industrial solar heat and solar district heating to offset the under-performing individual home segment. ince 2014 European concentrated solar power capacity for producing Selectricity has been more or less stable. New project constructions have been a long time coming, but this could change at the end of 2017 and in 2018 essentially in Italy. 51 millions m2 2 313.7 MWth The cumulated surfaces of solar thermal Total CSP capacity in operation Glenergy Solar in operation in the European Union in 2016 in the European Union in 2016 SOLAR THERMAL AND CONCENTRATED SOLAR POWER BAROMETERS – EUROBSERV’ER – JUIN 2017 SOLAR THERMAL AND CONCENTRATED SOLAR POWER BAROMETERS – EUROBSERV’ER – JUIN 2017 3 4 The world largest solar thermal Tabl. n° 1 district heating solution - Silkeborg, Denmark (in operation end 2016) Main solar thermal markets outside European Union Total cumulative capacity Annual Installed capacity (in MWth) in operation (in MWth) 2015 2016 2015 2016 China 30 500 27 664 309 500 337 164 United States 760 682 17 300 17 982 Turkey 1 500 1 467 13 600 15 067 India 770 894 6 300 7 194 Japan 100 50 2 400 2 450 Rest of the world 6 740 6 797 90 944 97 728 Total world 39 640 36 660 434 700 471 360 Source: EurObserv’ER 2017 new build, because of the construction is now causing great concern, where as a water production. -

Efficiency Gain of the Solar Trough Receiver Using Different Optically Active Layers
EFFICIENCY GAIN OF THE SOLAR TROUGH RECEIVER USING DIFFERENT OPTICALLY ACTIVE LAYERS Khaled Shehata Baiuomy Mohamad Supervised by: Professor Philippe Ferrer University of Witwatersrand Johannesburg, 24th October 2019 A dissertation submitted for the fulfillment of the requirements of the degree of Doctor of Philosophy to the Faculty of Science, University of Witwatersrand i Declaration I declare that this thesis is my own, unaided work. It is being submitted for the Degree of Doctor of Philosophy at the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination at any other University. Student: Khaled Shehata Baiuomy Mohamad Supervisor: Professor Philippe Ferrer ii Dedication To my family. iii Presentations arising from this study Paper Presentation, “Thermal performance analysis of novel alternative designs for parabolic trough solar collector,” 64th Annual Conference of the South African Institute of Physics (July 2019). (Granted an award for the best Ph.D. oral presentation in Applied Physics division) Paper Presentation, “Experimental and numerical study of a cavity and hot mirror receiver of the parabolic trough collector,” 63rd Annual Conference of the South African Institute of Physics (June 2018). Invited presentation, “Parabolic trough Efficiency gain through the use of a cavity absorber with a hot mirror,” Material and Energy research group, workshop, (November 2017). Invited Presentation, “Experimental and Numerical Heat Transfer Analysis of Cavity absorber and The Application of Different Optically Active Layer for Parabolic Solar Trough Concentrator,” Physics school, Wits University, (October 2017). Paper Presentation, “Experimental and Numerical Heat Transfer Analysis of Cavity absorber and The Application of Different Optically Active Layer for Parabolic Solar Trough Concentrator,” 62nd Annual Conference of the South African Institute of Physics (July 2017). -

Econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Nagl, Stephan; Fürsch, Michaela; Jägemann, Cosima; Bettzüge, Marc Oliver Working Paper The economic value of storage in renewable power systems - the case of thermal energy storage in concentrating solar plants EWI Working Paper, No. 11/08 Provided in Cooperation with: Institute of Energy Economics at the University of Cologne (EWI) Suggested Citation: Nagl, Stephan; Fürsch, Michaela; Jägemann, Cosima; Bettzüge, Marc Oliver (2011) : The economic value of storage in renewable power systems - the case of thermal energy storage in concentrating solar plants, EWI Working Paper, No. 11/08, Institute of Energy Economics at the University of Cologne (EWI), Köln This Version is available at: http://hdl.handle.net/10419/74392 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

3M Solar Technologies
3M Film Technologies Durable Films for Solar Light Management Tim Hebrink Staff Scientist 3M Company March 28, 2012 Photo courtesy of Ray Colby with Sundial Solar “Creating a brighter, more durable, more secure, and cleaner future” Hebrink Residence – Scandia Regional Lab 3M Film Technologies c-Si 3M Strategy for Solar Fabrication Reduced costs Thin Film Installation Cost Conversion kWh CPV Efficiency Light Management Increased energy Encapsulation output CSP Thermal management 3M Film Technologies 3M Solar Light Management Technologies Platforms Products Market Segments SMF 1100 Concentrated CSP Solar Power Electricity Generation Light Multilayer Industrial / Building Concentration Mirror Films Solar Thermal Heating Structural Low X Concentrated Panels CPV PhotoVoltaic Electricity Generation PhotoVoltaic and Surface Flat Panel Solar Thermal Structured Films/coatings Light Capture Weatherable Front Anti-Soil Thin Film and Back Surface Coatings Films Films Tapes Adhesives 3M Films/Tapes/Adhesives for c-Si Photovoltaic Junction Box Bonding Die cut of 3M™ Solar Acrylic Foam Tape or Bead of 3M™ PV 1000 Adhesive/Sealant Encapsulating 3M™ Scotchshield™ Film 17T (backside barrier film) Cosmetic Applications 3M™ Specialty Tapes Cell Positioning 3M™ Specialty Tapes Frame Bonding 3M™ Solar Acrylic Foam Tape Identification Solution 3M™ Performance Label Materials © 3M 2009. All Rights Reserved. 3M Film Technologies Multi-layer Optical Mirror Films ¼ Wave Constructive Interference Reflected waves – 100-1000 layers – 15-200 nm thick Incident wave – -
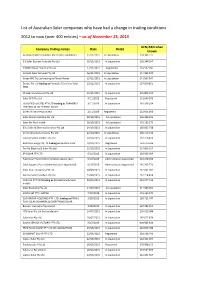
List of Australian Solar Companies Who Have Had a Change in Trading Conditions
List of Australian Solar companies who have had a change in trading conditions 2012 to now (over 400 entries) – as of November 25, 2015 ACN/ABN when Company trading names Date Detail known Australian Micro Inverters Pty Ltd (In Liquidation) 25/11/2015 In Liquidation 159 386 777 S.X Solar Express Australia Pty Ltd 19/11/2015 In Liquidation 126 348 507 CAIRNS VALUE SOLAR PTY LTD 17/11/2015 Registered 154 587 185 Armada Solar Services Pty Ltd 16/11/2015 In Liquidation 144 965 042 Green RRC Pty Ltd trading as Planet Power 13/11/2015 In Liquidation 141 584 947 Dorost Pty Ltd trading as Formerly T/as Clear Solar 12/11/2015 In Liquidation 137 609 851 NSW JP Solar Installations Pty Ltd 12/11/2015 In Liquidation 155 806 125 Solar DFO Pty Ltd 4/11/2015 Registered 151 646 203 QUALIFIED QUOTES PTY LTD trading as FORMERLY 3/11/2015 In Liquidation 165 356 294 TRADING AS NO DEPOSIT SOLAR Green Alliance Pty Limited 2/11/2015 Registered 122 821 363 Solar Winds Australia Pty Ltd 30/10/2015 In Liquidation 126 384 325 Solar Set Pty Limited 28/10/2015 In Liquidation 145 163 579 Elite Solar & Electrical Services Pty Ltd 24/10/2015 In Liquidation 166 865 738 Green Initiatives Services Pty Ltd 22/10/2015 In Liquidation 166 114 512 Sunrise Solar Installers Pty Ltd 20/10/2015 In Liquidation 132 218 850 Red Kite Energy Pty Ltd trading as Red Kite Solar 19/10/2015 Registered 163 525 344 Pro-Ro Electrics & Solar Pty Ltd 13/10/2015 In Liquidation 143 600 337 EP SOLAR PTY LTD 6/10/2015 In Liquidation 138 556 304 Free Solar Pty Ltd (Administrators Appointed) 6/10/2015 Administrators Appointed 131 248 336 Solar Support Pty Ltd (Administrators Appointed) 6/10/2015 Administrators Appointed 145 705 773 Solar City Enterprises Pty Ltd 30/09/2015 In Liquidation 142 641 281 Sunrise Solar Installers Pty Ltd 25/09/2015 In Liquidation 132 218 850 ACN 151 277 913 trading as PH Electrical & Solar 23/09/2015 In Liquidation 151 277 913 Installs Solar Economy Pty Ltd 14/09/2015 In Liquidation 144 963 959 SOLAR SET PTY LIMITED 7/09/2015 In Liquidation 145 163 579 SSW GROUP HOLDINGS PTY.