Best Practices for Reporting Electrocatalytic Performance of Nanomaterials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Copper Electroplating And
CHARACTERIZATION OF COPPER ELECTROPLATING AND ELECTROPOLISHING PROCESSES FOR SEMICONDUCTOR INTERCONNECT METALLIZATION by JULIE MARIE MENDEZ Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Dr. Uziel Landau Department of Chemical Engineering CASE WESTERN RESERVE UNIVERSITY August, 2009 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of _____________________________________________________ candidate for the ______________________degree *. (signed)_______________________________________________ (chair of the committee) ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ (date) _______________________ *We also certify that written approval has been obtained for any proprietary material contained therein. TABLE OF CONTENTS Page Number List of Tables 3 List of Figures 4 Acknowledgements 9 List of Symbols 10 Abstract 13 1. Introduction 15 1.1 Semiconductor Interconnect Metallization – Process Description 15 1.2 Mechanistic Aspects of Bottom-up Fill 20 1.3 Electropolishing 22 1.4 Topics Addressed in the Dissertation 24 2. Experimental Studies of Copper Electropolishing 26 2.1 Experimental Procedure 29 2.2 Polarization Studies 30 2.3 Current Steps 34 2.3.1 Current Stepped to a Level below Limiting Current 34 2.3.2 Current Stepped to the Limiting -

Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers
energies Review Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers Ana L. Santos 1,2, Maria-João Cebola 3,4,5 and Diogo M. F. Santos 2,* 1 TecnoVeritas—Serviços de Engenharia e Sistemas Tecnológicos, Lda, 2640-486 Mafra, Portugal; [email protected] 2 Center of Physics and Engineering of Advanced Materials (CeFEMA), Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisbon, Portugal 3 CBIOS—Center for Research in Biosciences & Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisbon, Portugal; [email protected] 4 CERENA—Centre for Natural Resources and the Environment, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon, Portugal 5 Escola Superior Náutica Infante D. Henrique, 2770-058 Paço de Arcos, Portugal * Correspondence: [email protected] Abstract: Environmental issues make the quest for better and cleaner energy sources a priority. Worldwide, researchers and companies are continuously working on this matter, taking one of two approaches: either finding new energy sources or improving the efficiency of existing ones. Hydrogen is a well-known energy carrier due to its high energy content, but a somewhat elusive one for being a gas with low molecular weight. This review examines the current electrolysis processes for obtaining hydrogen, with an emphasis on alkaline water electrolysis. This process is far from being new, but research shows that there is still plenty of room for improvement. The efficiency of an electrolyzer mainly relates to the overpotential and resistances in the cell. This work shows that the path to better Citation: Santos, A.L.; Cebola, M.-J.; electrolyzer efficiency is through the optimization of the cell components and operating conditions. -

Electrode Reactions and Kinetics
CHEM465/865, 2004-3, Lecture 14-16, 20 th Oct., 2004 Electrode Reactions and Kinetics From equilibrium to non-equilibrium : We have a cell, two individual electrode compartments, given composition, molar reaction Gibbs free ∆∆∆ energy as the driving force, rG , determining EMF Ecell (open circuit potential) between anode and cathode! In other words: everything is ready to let the current flow! – Make the external connection through wires. Current is NOT determined by equilibrium thermodynamics (i.e. by the composition of the reactant mixture, i.e. the reaction quotient Q), but it is controlled by resistances, activation barriers, etc Equilibrium is a limiting case – any kinetic model must give the correct equilibrium expressions). All the involved phenomena are generically termed ELECTROCHEMICAL KINETICS ! This involves: Bulk diffusion Ion migration (Ohmic resistance) Adsorption Charge transfer Desorption Let’s focus on charge transfer ! Also called: Faradaic process . The only reaction directly affected by potential! An electrode reaction differs from ordinary chemical reactions in that at least one partial reaction must be a charge transfer reaction – against potential-controlled activation energy, from one phase to another, across the electrical double layer. The reaction rate depends on the distributions of species (concentrations and pressures), temperature and electrode potential E. Assumption used in the following: the electrode material itself (metal) is inert, i.e. a catalyst. It is not undergoing any chemical transformation. It is only a container of electrons. The general question on which we will focus in the following is: How does reaction rate depend on potential? The electrode potential E of an electrode through which a current flows differs from the equilibrium potential Eeq established when no current flows. -

Lecture 03 Electrochemical Kinetics
EMA4303/5305 Electrochemical Engineering Lecture 03 Electrochemical Kinetics Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Electrochemical Kinetics Electrochemical kinetics “Electrochemical kinetics is a field of electrochemistry studying the rate of electrochemical processes. Due to electrochemical phenomena unfolding at the interface between an electrode and an electrolyte, there are accompanying phenomena to electrochemical reactions which contribute to the overall reaction rate.” (Wikipedia) “The main goal of the electrochemical kinetics is to find a relationship between the electrode overpotential and current density due to an applied potential.” (S. N. Lvov) Main contents of this lecture . Overpotential (η) . Charge transfer overpotential − Butler-Volmer equation − Tafel equation . Mass transfer overpotential EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 2 Cell Potential in Different Modes Electrolytic cell (EC) Galvanic cell (GC) 푬푬푸 = 푰 (푹풆풙풕 + 푹풊풏풕) 푬푨풑풑풍풊풆풅 = 푬푬푸 + 푰푹풊풏풕 푬풆풙풕 = 푬푬푸 − 푰푹풊풏풕= IRext EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 3 Cell Potential-Current Dependence . 푹풊풏풕 represents the total internal resistance of the cell, which is usually not zero, in most cases. Therefore, the difference between cell equilibrium potential and apparent potential is proportional to the current passing through the cell. Cell potential-current dependences EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 4 Overpotential (η) Overpotential for a single electrode . The difference between the actual potential (measured) for an electrode, E, and the equilibrium potential for that electrode, 푬푬푸, is called the overpotential of that electrode, 휼 = 푬 −푬푬푸, which could be either positive or negative, depending on the direction of the half (cell) reaction. -

Solar-Driven, Highly Sustained Splitting of Seawater Into Hydrogen and Oxygen Fuels
Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels Yun Kuanga,b,c,1, Michael J. Kenneya,1, Yongtao Menga,d,1, Wei-Hsuan Hunga,e, Yijin Liuf, Jianan Erick Huanga, Rohit Prasannag, Pengsong Lib,c, Yaping Lib,c, Lei Wangh,i, Meng-Chang Lind, Michael D. McGeheeg,j, Xiaoming Sunb,c,d,2, and Hongjie Daia,2 aDepartment of Chemistry, Stanford University, Stanford, CA 94305; bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China; cBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China; dCollege of Electrical Engineering and Automation, Shandong University of Science and Technology, Qingdao 266590, China; eDepartment of Materials Science and Engineering, Feng Chia University, Taichung 40724, Taiwan; fStanford Synchrotron Radiation Light Source, SLAC National Accelerator Laboratory, Menlo Park, CA 94025; gDepartment of Materials Science and Engineering, Stanford University, Stanford, CA 94305; hCenter for Electron Microscopy, Institute for New Energy Materials, Tianjin University of Technology, Tianjin 300384, China; iTianjin Key Laboratory of Advanced Functional Porous Materials, School of Materials, Tianjin University of Technology, Tianjin 300384, China; and jDepartment of Chemical Engineering, University of Colorado Boulder, Boulder, CO 80309 Contributed by Hongjie Dai, February 5, 2019 (sent for review January 14, 2019; reviewed by Xinliang Feng and Ali Javey) Electrolysis of water to generate hydrogen fuel is an attractive potential ∼490 mV higher than that of OER, which demands renewable energy storage technology. However, grid-scale fresh- highly active OER electrocatalysts capable of high-current (∼1 2 water electrolysis would put a heavy strain on vital water re- A/cm ) operations for high-rate H2/O2 production at over- sources. -

Overpotential Analysis of Alkaline and Acidic Alcohol Electrolysers And
1 2 Overpotential analysis of alkaline and acidic alcohol electrolysers 3 and optimized membrane-electrode assemblies 4 5 F. M. Sapountzia*, V. Di Palmab, G. Zafeiropoulosc, H. Penchevd, M.Α. Verheijenb, 6 M. Creatoreb, F. Ublekovd, V. Sinigerskyd, W.M. Arnold Bikc, H.O.A. Fredrikssona, 7 M.N. Tsampasc, J.W. Niemantsverdrieta 8 9 a SynCat@DIFFER, Syngaschem BV, P.O. Box 6336, 5600 HH, Eindhoven, The Netherlands, 10 www.syngaschem.com 11 b Department of Applied Physics, Eindhoven University of Technology, 5600MB, Eindhoven, The Netherlands 12 c DIFFER, Dutch Institute For Fundamental Energy Research, De Zaale 20, 5612 AJ, Eindhoven, The Netherlands 13 d Institute of Polymers, Bulgarian Academy of Sciences, Acad. Georgi Bonchev 103A Str., BG 1113, Sofia, 14 Bulgaria 15 16 *Corresponding author: Foteini Sapountzi, email: [email protected], postal address: Syngaschem BV, P.O. 17 Box 6336, 5600 HH, Eindhoven, The Netherlands 18 19 20 Abstract: Alcohol electrolysis using polymeric membrane electrolytes is a promising route for 21 storing excess renewable energy in hydrogen, alternative to the thermodynamically limited water 22 electrolysis. By properly choosing the ionic agent (i.e. H+ or OH-) and the catalyst support, and 23 by tuning the catalyst structure, we developed membrane-electrode-assemblies which are 24 suitable for cost-effective and efficient alcohol electrolysis. Novel porous electrodes were 25 prepared by Atomic Layer Deposition (ALD) of Pt on a TiO2-Ti web of microfibers and were 26 interfaced to polymeric membranes with either H+ or OH- conductivity. Our results suggest that 27 alcohol electrolysis is more efficient using OH- conducting membranes under appropriate 28 operation conditions (high pH in anolyte solution). -

Water Splitting by Heterogeneous Catalysis
!"#$ #"%"" &' () * ( +# ,% - . ( (.( ( . %/ .0! 1 ( % 2 3 . 4 . 5 % . . % . . . . . . 3 % !# 3 ( 3 ( 3 ( 3 %/. . - % . (. 6 7 % ( 2!0! . (/ (3 . 7 % ( ( 3 % . 3 ( %/ . 8 9 3 8 % ( :;0 9 . <= 3 9 . % ( . ( 3 > % !"#$ ?@@ %% @ A B ?? ? ? #;C#C# ,8D$CD#$$D$":D! ,8D$CD#$$D$";"C ! " # $ (#" D# WATER SPLITTING BY HETEROGENEOUS CATALYSIS Henrik Svengren Water splitting by heterogeneous catalysis Henrik Svengren ©Henrik Svengren, Stockholm University 2017 ISBN print 978-91-7797-039-2 ISBN PDF 978-91-7797-040-8 Printed in Sweden by Universitetsservice US-AB, Stockholm 2017 Distributed by the Department of Materials and Environmental Chemistry To my beloved family Cover image + Heterogeneous catalysis of water oxidation according to 2H2O ĺ O2 + 4H on CoSbO4/CoSb2O6, investigated in Paper II. The figure is composed of SEM- and TEM images, structural drawings, reactant- and product molecules. i Examination Faculty opponent Docent Tomas Edvinsson Solid State Physics Department of Engineering Sciences Uppsala University, -

Electrochemical Characterisation of Porous Cathodes in the Polymer Electrolyte Fuel Cell
ELECTROCHEMICAL CHARACTERISATION OF POROUS CATHODES IN THE POLYMER ELECTROLYTE FUEL CELL Frédéric Jaouen Doctoral Thesis Department of Chemical Engineering and Technology Applied Electrochemistry Kungl Tekniska Högskolan Stockholm 2003 TRITA-KET R175 ISSN 1104-3466 ISRN KTH/KET/R-175-SE ISBN 91-7283-450-1 ELECTROCHEMICAL CHARACTERISATION OF POROUS CATHODES IN THE POLYMER ELECTROLYTE FUEL CELL Frédéric Jaouen Doctoral Thesis Department of Chemical Engineering and Technology Applied Electrochemistry Kungl Tekniska Högskolan Stockholm 2003 AKADEMISK AVHANDLING som med tillstånd av Kungl Tekniska Högskolan i Stockholm, framlägges till offentlig granskning för avläggande av teknisk doktorsexamen fredagen den 25 april 2003, kl. 10.15 i Kollegiesalen, Valhallavägen 79, Kungl Tekniska Högskolan, Stockholm. Abstract Polymer electrolyte fuel cells (PEFC) convert chemical energy into electrical energy with higher efficiency than internal combustion engines. They are particularly suited for transportation applications or portable devices owing to their high power density and low operating temperature. The latter is however detrimental to the kinetics of electrochemical reactions and in particular to the reduction of oxygen at the cathode. The latter reaction requires enhancing by the very best catalyst, today platinum. Even so, the cathode is responsible for the main loss of voltage in the cell. Moreover, the scarce and expensive nature of platinum craves the optimisation of its use. The purpose of this thesis was to better understand the functioning of the porous cathode in the PEFC. This was achieved by developing physical models to predict the response of the cathode to steady-state polarisation, current interruption (CI) and electrochemical impedance spectroscopy (EIS), and by comparing these results to experimental ones. -

1 Electrochemical Kinetics of Corrosion and Passivity the Basis
Electrochemical Kinetics of Corrosion and Passivity The basis of a rate expression for an electrochemical process is Faraday’s law: Ita m = nF Where m is the mass reacted, I is the measured current in ampere, t is the time, a is the atomic weight, n the number of electrons transferred and F is the Faraday constant (96500 Cmol-1). Dividing Faraday’s law by the surface area A and the time t leads to an expression for the corrosion rate r: m ia r = = tA nF With the current density i defined as i = I/A. Exchange current density: We consider the reaction for the oxidation/reduction of hydrogen: rf + - 2H + 2e H2 rr 0 + This reaction is in the equilibrium state at the standard half cell potential e (H /H2). This means that the forward reaction rate rf and the reverse reaction rate rr have the same magnitude. This can be written as: i a r = r = 0 f r nF In this case is i0 the exchange current density equivalent to the reversible rate at equilibrium. In other words, while the standard half cell potential e0 is the universal thermodynamic parameter, i0 is the fundamental kinetic parameter of an electrochemical reaction. The exchange current density cannot be calculated. It has to be measured for each system. The following figure shows that the exchange current density for the hydrogen reaction depends strongly on the electrode material, whereas the standard half cell potential remains the same. 1 Electrochemical Polarization: Polarization η is the change in the standard half cell potential e caused by a net surface reaction rate. -

Understanding Anode Overpotential
UNDERSTANDING ANODE OVERPOTENTIAL Rebecca Jayne Thorne1, Camilla Sommerseth1, Ann Mari Svensson1, Espen Sandnes2, Lorentz Petter Lossius2, Hogne Linga2, and Arne Petter Ratvik1 1Dept. of Materials Science and Engineering, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway 2Hydro Aluminium, Årdal, Norway Keywords: Anode, carbon, electrochemistry, characterisation, overpotential. Abstract have not linked these coke properties to overpotential directly [2, 9-11]. It is likely that coke and anode physical properties will In aluminium electrolysis cells the anodic process is associated have an effect on overpotential, as the reactivity of carbons, both with a substantial overpotential. Industrial carbon anodes are electrochemical reactions and oxidation reactions, is generally produced from a blend of coke materials, but the effect of coke known to be related to microstructural parameters [12, 13]. In type on anodic overpotential has not been well studied. In this addition, the formation and release of CO2 bubbles also relies on work, lab-scale anodes were fabricated from single cokes and the surface microstructure [14]. electrochemical methods were subsequently used to determine the overpotential trend of the anode materials. Attempts were then In a previous study that served as preliminary work on a new made to explain these trends in terms of both the physical and project, the total overpotential of a range of carbon anodes chemical characteristics of the baked anodes themselves and their varying only in coke type was measured [15]. These anodes were raw materials. Routine coke and anode characterisation methods labeled 1 to 4, and a distinct overpotential trend was found with were used to measure properties such as impurity concentrations anode 4 having a lower overpotential than anode 1 by -2 and reactivity (to air and CO2), while non-routine characterisation approximately 200 mV at 1 A cm . -
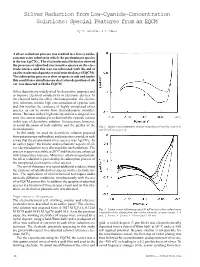
Silver Reduction from Low-Cyanide-Concentration Solutions: Special Features from an EQCM
Silver Reduction from Low-Cyanide-Concentration Solutions: Special Features from an EQCM By H. Sanchez & Y. Meas A silver reduction process was studied in a low-cyanide- concentration solution in which the predominant species - is the ion Ag(CN)2 . The electrochemical behavior showed the presence of adsorbed electroactive species on the elec- trode surface and this was corroborated with the aid of an electrochemical quartz crystal microbalance (EQCM). The adsorption process is slow at open circuit and under this condition a simultaneous electroless deposition of sil- ver was detected with the EQCM. Silver deposits are widely used for decorative purposes and to improve electrical conductivity in electronic devices. In the classical baths for silver electrodeposition, the electro- lytic solutions contain high concentrations of cyanide ions and this implies the existence of highly complexed silver species, as can be shown from thermodynamic consider- ations.1 Because of their high toxicity and for ecological rea- sons, the current tendency is to diminish the cyanide content in this type of electrolytic solution. It is necessary, however, to avoid alteration of bath stability and the quality of the Fig. 1—Single scan voltammetry of silver reduction at 10 mV/sec (curve 1) electrodeposits. and 20 mV/sec (curve 2). In this study, we used an electrolytic solution prepared from potassium pyrophosphate and potassium cyanide in such - a way that the predominant silver species was Ag(CN)2 . In an earlier paper,1 the kinetic and mechanistic aspects of sil- ver electroreduction were discussed for such solutions. The process is quasi-reversible at 20 °C and kinetics are enhanced with temperature increase. -

An Investigation Into Aqueous Titanium
An Investigation into Aqueous Titanium Speciation Utilising Electrochemical Methods for the Purpose of Implementation into the Sulfate Process for Titanium Dioxide Manufacture Samala Shepherd, BSc. (Hons) Masters of Philosophy in Chemistry University of Newcastle March, 2013 STATEMENT OF ORIGINALITY This thesis contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. I give consent to this copy of my thesis, when deposited in the University Library**, being made available for loan and photocopying subject to the provisions of the Copyright Act 1968. **Unless an Embargo has been approved for a determined period. Samala L. Shepherd i Acknowledgements There are many people who have helped me and contributed to my work in a number of ways and I’d like to thank them. I’d like to thank BHP Billiton Newcastle Technology Centre for making this project possible. The ARC for support and funding. Dr. Scott Donne for the vast knowledge he provided me with and the friendship and support. Thank you to Carolyn Freeburn, Vicki Thompson, and Stephen Hopkins for the ‘store/equipment room’ when I was in need. Thank you to Dianna Brennan for keeping me supplied. Michael Fitzgerald for his continued support. Last but not least a big thanks to my family, they are stuck with me but bare the burden with smiles and support and I thank them greatly.