Role of Diffuse Layer in Acidic and Alkaline Fuel Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers
energies Review Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers Ana L. Santos 1,2, Maria-João Cebola 3,4,5 and Diogo M. F. Santos 2,* 1 TecnoVeritas—Serviços de Engenharia e Sistemas Tecnológicos, Lda, 2640-486 Mafra, Portugal; [email protected] 2 Center of Physics and Engineering of Advanced Materials (CeFEMA), Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisbon, Portugal 3 CBIOS—Center for Research in Biosciences & Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisbon, Portugal; [email protected] 4 CERENA—Centre for Natural Resources and the Environment, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049-001 Lisbon, Portugal 5 Escola Superior Náutica Infante D. Henrique, 2770-058 Paço de Arcos, Portugal * Correspondence: [email protected] Abstract: Environmental issues make the quest for better and cleaner energy sources a priority. Worldwide, researchers and companies are continuously working on this matter, taking one of two approaches: either finding new energy sources or improving the efficiency of existing ones. Hydrogen is a well-known energy carrier due to its high energy content, but a somewhat elusive one for being a gas with low molecular weight. This review examines the current electrolysis processes for obtaining hydrogen, with an emphasis on alkaline water electrolysis. This process is far from being new, but research shows that there is still plenty of room for improvement. The efficiency of an electrolyzer mainly relates to the overpotential and resistances in the cell. This work shows that the path to better Citation: Santos, A.L.; Cebola, M.-J.; electrolyzer efficiency is through the optimization of the cell components and operating conditions. -

Electrode Reactions and Kinetics
CHEM465/865, 2004-3, Lecture 14-16, 20 th Oct., 2004 Electrode Reactions and Kinetics From equilibrium to non-equilibrium : We have a cell, two individual electrode compartments, given composition, molar reaction Gibbs free ∆∆∆ energy as the driving force, rG , determining EMF Ecell (open circuit potential) between anode and cathode! In other words: everything is ready to let the current flow! – Make the external connection through wires. Current is NOT determined by equilibrium thermodynamics (i.e. by the composition of the reactant mixture, i.e. the reaction quotient Q), but it is controlled by resistances, activation barriers, etc Equilibrium is a limiting case – any kinetic model must give the correct equilibrium expressions). All the involved phenomena are generically termed ELECTROCHEMICAL KINETICS ! This involves: Bulk diffusion Ion migration (Ohmic resistance) Adsorption Charge transfer Desorption Let’s focus on charge transfer ! Also called: Faradaic process . The only reaction directly affected by potential! An electrode reaction differs from ordinary chemical reactions in that at least one partial reaction must be a charge transfer reaction – against potential-controlled activation energy, from one phase to another, across the electrical double layer. The reaction rate depends on the distributions of species (concentrations and pressures), temperature and electrode potential E. Assumption used in the following: the electrode material itself (metal) is inert, i.e. a catalyst. It is not undergoing any chemical transformation. It is only a container of electrons. The general question on which we will focus in the following is: How does reaction rate depend on potential? The electrode potential E of an electrode through which a current flows differs from the equilibrium potential Eeq established when no current flows. -

Lecture 03 Electrochemical Kinetics
EMA4303/5305 Electrochemical Engineering Lecture 03 Electrochemical Kinetics Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Electrochemical Kinetics Electrochemical kinetics “Electrochemical kinetics is a field of electrochemistry studying the rate of electrochemical processes. Due to electrochemical phenomena unfolding at the interface between an electrode and an electrolyte, there are accompanying phenomena to electrochemical reactions which contribute to the overall reaction rate.” (Wikipedia) “The main goal of the electrochemical kinetics is to find a relationship between the electrode overpotential and current density due to an applied potential.” (S. N. Lvov) Main contents of this lecture . Overpotential (η) . Charge transfer overpotential − Butler-Volmer equation − Tafel equation . Mass transfer overpotential EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 2 Cell Potential in Different Modes Electrolytic cell (EC) Galvanic cell (GC) 푬푬푸 = 푰 (푹풆풙풕 + 푹풊풏풕) 푬푨풑풑풍풊풆풅 = 푬푬푸 + 푰푹풊풏풕 푬풆풙풕 = 푬푬푸 − 푰푹풊풏풕= IRext EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 3 Cell Potential-Current Dependence . 푹풊풏풕 represents the total internal resistance of the cell, which is usually not zero, in most cases. Therefore, the difference between cell equilibrium potential and apparent potential is proportional to the current passing through the cell. Cell potential-current dependences EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 4 Overpotential (η) Overpotential for a single electrode . The difference between the actual potential (measured) for an electrode, E, and the equilibrium potential for that electrode, 푬푬푸, is called the overpotential of that electrode, 휼 = 푬 −푬푬푸, which could be either positive or negative, depending on the direction of the half (cell) reaction. -

Solar-Driven, Highly Sustained Splitting of Seawater Into Hydrogen and Oxygen Fuels
Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels Yun Kuanga,b,c,1, Michael J. Kenneya,1, Yongtao Menga,d,1, Wei-Hsuan Hunga,e, Yijin Liuf, Jianan Erick Huanga, Rohit Prasannag, Pengsong Lib,c, Yaping Lib,c, Lei Wangh,i, Meng-Chang Lind, Michael D. McGeheeg,j, Xiaoming Sunb,c,d,2, and Hongjie Daia,2 aDepartment of Chemistry, Stanford University, Stanford, CA 94305; bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China; cBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China; dCollege of Electrical Engineering and Automation, Shandong University of Science and Technology, Qingdao 266590, China; eDepartment of Materials Science and Engineering, Feng Chia University, Taichung 40724, Taiwan; fStanford Synchrotron Radiation Light Source, SLAC National Accelerator Laboratory, Menlo Park, CA 94025; gDepartment of Materials Science and Engineering, Stanford University, Stanford, CA 94305; hCenter for Electron Microscopy, Institute for New Energy Materials, Tianjin University of Technology, Tianjin 300384, China; iTianjin Key Laboratory of Advanced Functional Porous Materials, School of Materials, Tianjin University of Technology, Tianjin 300384, China; and jDepartment of Chemical Engineering, University of Colorado Boulder, Boulder, CO 80309 Contributed by Hongjie Dai, February 5, 2019 (sent for review January 14, 2019; reviewed by Xinliang Feng and Ali Javey) Electrolysis of water to generate hydrogen fuel is an attractive potential ∼490 mV higher than that of OER, which demands renewable energy storage technology. However, grid-scale fresh- highly active OER electrocatalysts capable of high-current (∼1 2 water electrolysis would put a heavy strain on vital water re- A/cm ) operations for high-rate H2/O2 production at over- sources. -

Overpotential Analysis of Alkaline and Acidic Alcohol Electrolysers And
1 2 Overpotential analysis of alkaline and acidic alcohol electrolysers 3 and optimized membrane-electrode assemblies 4 5 F. M. Sapountzia*, V. Di Palmab, G. Zafeiropoulosc, H. Penchevd, M.Α. Verheijenb, 6 M. Creatoreb, F. Ublekovd, V. Sinigerskyd, W.M. Arnold Bikc, H.O.A. Fredrikssona, 7 M.N. Tsampasc, J.W. Niemantsverdrieta 8 9 a SynCat@DIFFER, Syngaschem BV, P.O. Box 6336, 5600 HH, Eindhoven, The Netherlands, 10 www.syngaschem.com 11 b Department of Applied Physics, Eindhoven University of Technology, 5600MB, Eindhoven, The Netherlands 12 c DIFFER, Dutch Institute For Fundamental Energy Research, De Zaale 20, 5612 AJ, Eindhoven, The Netherlands 13 d Institute of Polymers, Bulgarian Academy of Sciences, Acad. Georgi Bonchev 103A Str., BG 1113, Sofia, 14 Bulgaria 15 16 *Corresponding author: Foteini Sapountzi, email: [email protected], postal address: Syngaschem BV, P.O. 17 Box 6336, 5600 HH, Eindhoven, The Netherlands 18 19 20 Abstract: Alcohol electrolysis using polymeric membrane electrolytes is a promising route for 21 storing excess renewable energy in hydrogen, alternative to the thermodynamically limited water 22 electrolysis. By properly choosing the ionic agent (i.e. H+ or OH-) and the catalyst support, and 23 by tuning the catalyst structure, we developed membrane-electrode-assemblies which are 24 suitable for cost-effective and efficient alcohol electrolysis. Novel porous electrodes were 25 prepared by Atomic Layer Deposition (ALD) of Pt on a TiO2-Ti web of microfibers and were 26 interfaced to polymeric membranes with either H+ or OH- conductivity. Our results suggest that 27 alcohol electrolysis is more efficient using OH- conducting membranes under appropriate 28 operation conditions (high pH in anolyte solution). -

Understanding Anode Overpotential
UNDERSTANDING ANODE OVERPOTENTIAL Rebecca Jayne Thorne1, Camilla Sommerseth1, Ann Mari Svensson1, Espen Sandnes2, Lorentz Petter Lossius2, Hogne Linga2, and Arne Petter Ratvik1 1Dept. of Materials Science and Engineering, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway 2Hydro Aluminium, Årdal, Norway Keywords: Anode, carbon, electrochemistry, characterisation, overpotential. Abstract have not linked these coke properties to overpotential directly [2, 9-11]. It is likely that coke and anode physical properties will In aluminium electrolysis cells the anodic process is associated have an effect on overpotential, as the reactivity of carbons, both with a substantial overpotential. Industrial carbon anodes are electrochemical reactions and oxidation reactions, is generally produced from a blend of coke materials, but the effect of coke known to be related to microstructural parameters [12, 13]. In type on anodic overpotential has not been well studied. In this addition, the formation and release of CO2 bubbles also relies on work, lab-scale anodes were fabricated from single cokes and the surface microstructure [14]. electrochemical methods were subsequently used to determine the overpotential trend of the anode materials. Attempts were then In a previous study that served as preliminary work on a new made to explain these trends in terms of both the physical and project, the total overpotential of a range of carbon anodes chemical characteristics of the baked anodes themselves and their varying only in coke type was measured [15]. These anodes were raw materials. Routine coke and anode characterisation methods labeled 1 to 4, and a distinct overpotential trend was found with were used to measure properties such as impurity concentrations anode 4 having a lower overpotential than anode 1 by -2 and reactivity (to air and CO2), while non-routine characterisation approximately 200 mV at 1 A cm . -

Section 3.4 Fuel Cells
2016 FUEL CELLS SECTION 3.4 Fuel Cells Fuel cells efficiently convert diverse fuels directly into electricity without combustion, and they are key elements of a broad portfolio for building a competitive, secure, and sustainable clean energy economy. They offer a broad range of benefits, including reduced greenhouse gas emissions; reduced oil consumption; expanded use of renewable power (through the use of hydrogen derived from renewable resources as a transportation fuel as well as for energy storage and transmission); highly efficient energy conversion; fuel flexibility (use of diverse, domestic fuels, including hydrogen, natural gas, biogas, and methanol); reduced air pollution, criteria pollutants, water use; and highly reliable grid support. Fuel cells also have numerous advantages that make them appealing for end users, including quiet operation, low maintenance needs, and high reliability. Because of their broad applicability and diverse uses, fuel cells can address critical challenges in all energy sectors: commercial, residential, industrial, and transportation. The fuel cell industry had revenues of approximately $2.2 billion in 2014, an increase of almost $1 billion over revenues in 2013.1 The largest markets for fuel cells today are in stationary power, portable power, auxiliary power units, backup power, and material handling equipment. Approximately 155,000 fuel cells were shipped worldwide in the four-year period from 2010 through 2013, accounting for 510–583 MW of fuel cell capacity.2 In 2014 alone, more than 50,000 fuel cells accounting for over 180 MW of capacity were shipped.1 In transportation applications, manufacturers have begun to commercialize fuel cell electric vehicles (FCEVs). Hyundai and Toyota have recently introduced their FCEVs in the marketplace, and Honda is set to launch its new FCEV in the market in 2016. -
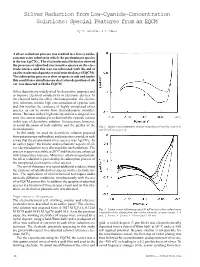
Silver Reduction from Low-Cyanide-Concentration Solutions: Special Features from an EQCM
Silver Reduction from Low-Cyanide-Concentration Solutions: Special Features from an EQCM By H. Sanchez & Y. Meas A silver reduction process was studied in a low-cyanide- concentration solution in which the predominant species - is the ion Ag(CN)2 . The electrochemical behavior showed the presence of adsorbed electroactive species on the elec- trode surface and this was corroborated with the aid of an electrochemical quartz crystal microbalance (EQCM). The adsorption process is slow at open circuit and under this condition a simultaneous electroless deposition of sil- ver was detected with the EQCM. Silver deposits are widely used for decorative purposes and to improve electrical conductivity in electronic devices. In the classical baths for silver electrodeposition, the electro- lytic solutions contain high concentrations of cyanide ions and this implies the existence of highly complexed silver species, as can be shown from thermodynamic consider- ations.1 Because of their high toxicity and for ecological rea- sons, the current tendency is to diminish the cyanide content in this type of electrolytic solution. It is necessary, however, to avoid alteration of bath stability and the quality of the Fig. 1—Single scan voltammetry of silver reduction at 10 mV/sec (curve 1) electrodeposits. and 20 mV/sec (curve 2). In this study, we used an electrolytic solution prepared from potassium pyrophosphate and potassium cyanide in such - a way that the predominant silver species was Ag(CN)2 . In an earlier paper,1 the kinetic and mechanistic aspects of sil- ver electroreduction were discussed for such solutions. The process is quasi-reversible at 20 °C and kinetics are enhanced with temperature increase. -

Iron Based Cathode Catalyst for Alkaline Fuel Cells Thesis Presented in Partial Fulfillment of the Requirements for Honors Resea
Iron Based Cathode Catalyst for Alkaline Fuel Cells Thesis Presented in Partial Fulfillment of the Requirements for Honors Research Distinction at The Ohio State University By Christopher Bruening Undergraduate Program in Chemical Engineering The Ohio State University 2014 Thesis Committee: Dr. Umit Ozkan, Advisor Dr. Kurt Koelling Copyright by Christopher Bruening 2014 Abstract Alkaline fuel cells take advantage of the reaction between hydrogen and oxygen to produce water and an electrical current. During the reaction, a hydroxide ion passes through a membrane from the oxygen side cathode to the hydrogen side anode. Previous research had been conducted on synthesis and evaluation of iron based catalysts for PEM fuel cells by the Heterogeneous Catalysis Research Group at The Ohio State University. PEM fuel cells use a similar reaction between hydrogen and oxygen except a proton travels across the membrane from the hydrogen side to the oxygen side, so the reaction mechanism differs. This paper looks into those same iron based catalysts tested in an alkaline environment. The catalyst in particular is 1% iron in 1:1 Black Pearls 2000 : 1,10 phenanthroline. Those precursors are combined using a wet impregnation and then the result is ball milled. It then undergoes an argon pyrolysis at 1050oC and an ammonia pyrolysis at 950oC which are the final steps in the synthesis. The completed catalyst can then be washed in acid for 1 hour, 2 days, or 1 week to compare the effect of acid washing on different factors. The different catalysts are compared at each stage in the synthesis mainly by their onset potential, which is the voltage where it produces a current density of 0.1 mA/cm2. -

Advanced Ionomers & Meas for Alkaline Membrane Fuel Cells
Advanced Ionomers & MEAs for Alkaline Membrane Fuel Cells PI Bryan Pivovar National Renewable Energy Laboratory June 15, 2018 DOE Hydrogen and Fuel Cells Program 2018 Annual Merit Review and Peer Evaluation Meeting Project ID #FC147 This presentation does not contain any proprietary, confidential, or otherwise restricted information. Overview Timeline and Budget Barriers • Project start: October 2015 • Durability • Project end: March 2019 • Cost • % complete: ~ 70% • Performance • DOE Budget plan Partners – FY 2016 - 2018 $ 2,600k – Cost Share Percentage – 0% • LBNL – Adam Weber • ORNL/UTK – Tom Zawodzinski • Colorado School of Mines – Andy Herring • (in-kind) 3M – Mike Yandrasits NREL | 2 Relevance/Impact DOE (Preliminary) Milestones for AMFCs* • Q2, 2017: Develop anion-exchange membranes with an area specific resistance ≤ 0.1 ohm cm2, maintained for 500 hours during testing at 600 mA/cm2 at T >60 oC. • Q4, 2017: Demonstrate alkaline membrane fuel cell peak power performance > 600 mW/cm2 on 2 H2/O2 (maximum pressure of 1.5 atma) in MEA with a total loading of ≤ 0.125 mgPGM/cm . • Q2, 2019: Demonstrate alkaline membrane fuel cell initial performance of 0.6 V at 600 mA/cm2 on 2 H2/air (maximum pressure of 1.5 atma) in MEA a total loading of < 0.1 mgPGM/cm , and less than 10% voltage degradation over 2,000 hour hold test at 600 mA/cm2 at T>60 oC. Cell may be reconditioned during test to remove recoverable performance losses. • Q2, 2020: Develop non-PGM catalysts demonstrating alkaline membrane fuel cell peak power performance > 600 mW/cm2 under hydrogen/air (maximum pressure of 1.5 atma) in PGM-free MEA. -

Development of Power Generation by Durect Ethanol Fuel Cell
FUEL CELL SYSTEMS S. Basu New Delhi Department of Chemical Engineering Indian Institute of Technology Delhi INDIA e- Load Water 1. Fuel chamber 2. Oxidant chamber 1 4 2 3. Anode (Pt) 4. Electrolyte Fuel 3 5 Oxidant 5. Cathode (Pt/C) (H ) 2 O2/Air + - H2 2 H +2e (Anode) + - 2 H +1/2 O2 +2e H2O (Cathode) H2 + 1/2 O2 H2O (Overall) 9 Automobile 9 Efficient Power Generation 9 Distributed Power Gen. 9 Environmental Friendly 9 Portable Electronics Eqpt. Fuel Cell Families Phosphoric Acid Cathode Anode o H+ 220 C Solid Polymer Cathode Anode o o H+ 30 C- 80 C Alkaline Air Cathode - Anode Fuel 50 oC- 200 oC OH Molten Carbonates o Cathode = Anode 650 C CO3 Solid Oxides Cathode Anode o o O= 700 C – 1000 C Alkaline Fuel Cell ALKALINE FUEL CELL Alkaline electrolyte - - Anode H2 + 2OH Æ 2H2O + 2e - - Cathode 1/2O2 + H2O + 2e Æ 2OH Overall H2 + 1/2O2 Æ H2O + electrical Energy +heat Poisoning: - 2- CO2 +2OH (CO3) + H2O ¾ Depletion of KOH ¾ Poisoning of cathode surface with carbonates Myth ! 9Kordesch, K. et al., (1999), Intermittent Use of a Low-Cost Alkaline Fuel Cell-hybrid System for Electric Vehicles, J. Power Sources, Vol. 80, pp. 9McLean, G. F. et al. (2002) An assessment of alkaline fuel cell technology, Int. J. Hydrogen Energy 27 507 9Gülzow, E., and Schulze, M. (2003), Long-Term Operation of AFC Electrodes With CO2- Containing Gases, J. Power Sources, Polymer Electrolyte Membrane Fuel Cell (PEMFC) Hydrogen Fuel Cell Direct Alcohol Fuel Cell (DAFC) Fuel Processor Fuel Cleaning System Fuel Pure H2 Electricity Fuel Cell Direct Methanol Fuel Cell Direct Ethanol Fuel Cell (DMFC) (DEFC) Reduce CO adsorption on Pt at High Temp Higher power density at high temperature Easy breakage C-C bond in DEFC at High Temp. -

Mechanism of the Chlorine Evolution on a Ruthenium Oxide/Titanium Oxide Electrode and on a Ruthenium Electrode
Mechanism of the chlorine evolution on a ruthenium oxide/titanium oxide electrode and on a ruthenium electrode Citation for published version (APA): Janssen, L. J. J., Starmans, L. M. C., Visser, J. G., & Barendrecht, E. (1977). Mechanism of the chlorine evolution on a ruthenium oxide/titanium oxide electrode and on a ruthenium electrode. Electrochimica Acta, 22(10), 1093-1100. https://doi.org/10.1016/0013-4686(77)80045-5 DOI: 10.1016/0013-4686(77)80045-5 Document status and date: Published: 01/01/1977 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research.