Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrode Reactions and Kinetics
CHEM465/865, 2004-3, Lecture 14-16, 20 th Oct., 2004 Electrode Reactions and Kinetics From equilibrium to non-equilibrium : We have a cell, two individual electrode compartments, given composition, molar reaction Gibbs free ∆∆∆ energy as the driving force, rG , determining EMF Ecell (open circuit potential) between anode and cathode! In other words: everything is ready to let the current flow! – Make the external connection through wires. Current is NOT determined by equilibrium thermodynamics (i.e. by the composition of the reactant mixture, i.e. the reaction quotient Q), but it is controlled by resistances, activation barriers, etc Equilibrium is a limiting case – any kinetic model must give the correct equilibrium expressions). All the involved phenomena are generically termed ELECTROCHEMICAL KINETICS ! This involves: Bulk diffusion Ion migration (Ohmic resistance) Adsorption Charge transfer Desorption Let’s focus on charge transfer ! Also called: Faradaic process . The only reaction directly affected by potential! An electrode reaction differs from ordinary chemical reactions in that at least one partial reaction must be a charge transfer reaction – against potential-controlled activation energy, from one phase to another, across the electrical double layer. The reaction rate depends on the distributions of species (concentrations and pressures), temperature and electrode potential E. Assumption used in the following: the electrode material itself (metal) is inert, i.e. a catalyst. It is not undergoing any chemical transformation. It is only a container of electrons. The general question on which we will focus in the following is: How does reaction rate depend on potential? The electrode potential E of an electrode through which a current flows differs from the equilibrium potential Eeq established when no current flows. -

Nitrogen-Rich Graphitic-Carbon@Graphene As A
www.nature.com/scientificreports OPEN Nitrogen‑rich graphitic‑carbon@ graphene as a metal‑free electrocatalyst for oxygen reduction reaction Halima Begum, Mohammad Shamsuddin Ahmed & Young‑Bae Kim* The metal‑free nitrogen‑doped graphitic‑carbon@graphene (Ng‑c@G) is prepared from a composite of polyaniline and graphene by a facile polymerization following by pyrolysis for electrochemical oxygen reduction reaction (ORR). Pyrolysis creates a sponge‑like with ant‑cave‑architecture in the polyaniline derived nitrogenous graphitic‑carbon on graphene. The nitrogenous carbon is highly graphitized and most of the nitrogen atoms are in graphitic and pyridinic forms with less oxygenated is found when pyrolyzed at 800 °C. The electrocatalytic activity of Ng-C@G-800 is even better than the benchmarked Pt/C catalyst resulting in the higher half-wave potential (8 mV) and limiting current density (0.74 mA cm−2) for ORR in alkaline medium. Higher catalytic performance is originated from the special porous structure at microscale level and the abundant graphitic‑ and pyridinic‑n active sites at the nanoscale level on carbon-graphene matrix which are benefcial to the high O2‑mass transportation to those accessible sites. Also, it possesses a higher cycle stability resulting in the negligible potential shift and slight oxidation of pyridinic‑n with better tolerance to the methanol. To save the world from day-by-day increasing energy demands and environmental concerns, the clean, highly efcient and renewable energy technologies are immediately required to be implemented 1. Among various renewable energy technologies, fuel cells (FCs) and metal-air batteries are regarded as the promising clean energy sources because of their high energy conversion efciency and emission-free power generation 2,3. -

Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC)
applied sciences Review Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC) Nabila A. Karim 1,* and Hsiharng Yang 2,3,* 1 Fuel Cell Institute, Universiti Kebangsaan Malaysia, Bangi 43600, Selangor, Malaysia 2 Graduate Institute of Precision Engineering, National Chung Hsing University, 145 Xingda Road, South District, Taichung City 402, Taiwan 3 Innovation and Development Center of Sustainable Agriculture (IDCSA), National Chung Hsing University, 145 Xingda Road, South District, Taichung City 402, Taiwan * Correspondence: [email protected] (N.A.K.); [email protected] (H.Y.) Abstract: Enzymatic biofuel cells (EBFCs) is one of the branches of fuel cells that can provide high potential for various applications. However, EBFC has challenges in improving the performance power output. Exploring electrode materials is one way to increase enzyme utilization and lead to a high conversion rate so that efficient enzyme loading on the electrode surface can function correctly. This paper briefly presents recent technologies developed to improve bio-catalytic proper- ties, biocompatibility, biodegradability, implantability, and mechanical flexibility in EBFCs. Among the combinations of materials that can be studied and are interesting because of their properties, there are various nanoparticles, carbon-based materials, and conductive polymers; all three have the advantages of chemical stability and enhanced electron transfer. The methods to immobilize enzymes, and support and substrate issues are also covered in this paper. In addition, the EBFC system is also explored and developed as suitable for applications such as self-pumping and microfluidic EBFC. Citation: A. Karim, N.; Yang, H. Keywords: electrode; support; immobilization; enzyme; EBFC Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC). -

Steering CO2 Electroreduction Toward Ethanol Production by a Surface-Bound Ru Polypyridyl Carbene Catalyst on N-Doped Porous Carbon
Steering CO2 electroreduction toward ethanol production by a surface-bound Ru polypyridyl carbene catalyst on N-doped porous carbon Yanming Liua,b, Xinfei Fanc, Animesh Nayakb, Ying Wangb, Bing Shanb, Xie Quana, and Thomas J. Meyerb,1 aKey Laboratory of Industrial Ecology and Environmental Engineering, Ministry of Education, School of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, China; bDepartment of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; and cCollege of Environmental Science and Engineering, Dalian Maritime University, Dalian 116024, China Contributed by Thomas J. Meyer, November 10, 2019 (sent for review May 6, 2019; reviewed by Andrew B. Bocarsly and Clifford P. Kubiak) Electrochemical reduction of CO2 to multicarbon products is a sig- necessitates coupling reactions between a CO intermediate and/or nificant challenge, especially for molecular complexes. We report intermediates from CO protonation (29–31). Assembling the Ru(II) here CO2 reduction to multicarbon products based on a Ru(II) poly- polypyridyl carbene on an electrode surface that is capable of pyridyl carbene complex that is immobilized on an N-doped porous C–C dimerization offers an attractive strategy for reducing CO2 carbon (RuPC/NPC) electrode. The catalyst utilizes the synergistic to multicarbon products. effects of the Ru(II) polypyridyl carbene complex and the NPC N-doped carbon nanomaterials have been widely used for interface to steer CO2 reduction toward C2 production at low electroreduction due to their electrocatalytic activity and low cost. overpotentials. In 0.5 M KHCO3/CO2 aqueous solutions, Faradaic N-doped carbon electrodes have been shown to be capable of C–C efficiencies of 31.0 to 38.4% have been obtained for C2 production dimerization (6, 32). -

Lecture 03 Electrochemical Kinetics
EMA4303/5305 Electrochemical Engineering Lecture 03 Electrochemical Kinetics Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Electrochemical Kinetics Electrochemical kinetics “Electrochemical kinetics is a field of electrochemistry studying the rate of electrochemical processes. Due to electrochemical phenomena unfolding at the interface between an electrode and an electrolyte, there are accompanying phenomena to electrochemical reactions which contribute to the overall reaction rate.” (Wikipedia) “The main goal of the electrochemical kinetics is to find a relationship between the electrode overpotential and current density due to an applied potential.” (S. N. Lvov) Main contents of this lecture . Overpotential (η) . Charge transfer overpotential − Butler-Volmer equation − Tafel equation . Mass transfer overpotential EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 2 Cell Potential in Different Modes Electrolytic cell (EC) Galvanic cell (GC) 푬푬푸 = 푰 (푹풆풙풕 + 푹풊풏풕) 푬푨풑풑풍풊풆풅 = 푬푬푸 + 푰푹풊풏풕 푬풆풙풕 = 푬푬푸 − 푰푹풊풏풕= IRext EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 3 Cell Potential-Current Dependence . 푹풊풏풕 represents the total internal resistance of the cell, which is usually not zero, in most cases. Therefore, the difference between cell equilibrium potential and apparent potential is proportional to the current passing through the cell. Cell potential-current dependences EMA 5305 Electrochemical Engineering Zhe Cheng (2017) 3 Electrochemical Kinetics 4 Overpotential (η) Overpotential for a single electrode . The difference between the actual potential (measured) for an electrode, E, and the equilibrium potential for that electrode, 푬푬푸, is called the overpotential of that electrode, 휼 = 푬 −푬푬푸, which could be either positive or negative, depending on the direction of the half (cell) reaction. -

Solar-Driven, Highly Sustained Splitting of Seawater Into Hydrogen and Oxygen Fuels
Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels Yun Kuanga,b,c,1, Michael J. Kenneya,1, Yongtao Menga,d,1, Wei-Hsuan Hunga,e, Yijin Liuf, Jianan Erick Huanga, Rohit Prasannag, Pengsong Lib,c, Yaping Lib,c, Lei Wangh,i, Meng-Chang Lind, Michael D. McGeheeg,j, Xiaoming Sunb,c,d,2, and Hongjie Daia,2 aDepartment of Chemistry, Stanford University, Stanford, CA 94305; bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China; cBeijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China; dCollege of Electrical Engineering and Automation, Shandong University of Science and Technology, Qingdao 266590, China; eDepartment of Materials Science and Engineering, Feng Chia University, Taichung 40724, Taiwan; fStanford Synchrotron Radiation Light Source, SLAC National Accelerator Laboratory, Menlo Park, CA 94025; gDepartment of Materials Science and Engineering, Stanford University, Stanford, CA 94305; hCenter for Electron Microscopy, Institute for New Energy Materials, Tianjin University of Technology, Tianjin 300384, China; iTianjin Key Laboratory of Advanced Functional Porous Materials, School of Materials, Tianjin University of Technology, Tianjin 300384, China; and jDepartment of Chemical Engineering, University of Colorado Boulder, Boulder, CO 80309 Contributed by Hongjie Dai, February 5, 2019 (sent for review January 14, 2019; reviewed by Xinliang Feng and Ali Javey) Electrolysis of water to generate hydrogen fuel is an attractive potential ∼490 mV higher than that of OER, which demands renewable energy storage technology. However, grid-scale fresh- highly active OER electrocatalysts capable of high-current (∼1 2 water electrolysis would put a heavy strain on vital water re- A/cm ) operations for high-rate H2/O2 production at over- sources. -

Electrocatalyst Development for PEM Water Electrolysis and DMFC: Towards the Methanol Economy
Electrocatalyst development for PEM water electrolysis and DMFC: towards the methanol economy Radostina Vasileva Genova-Koleva ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR o al Repositorio Digital de la UB. -

Overpotential Analysis of Alkaline and Acidic Alcohol Electrolysers And
1 2 Overpotential analysis of alkaline and acidic alcohol electrolysers 3 and optimized membrane-electrode assemblies 4 5 F. M. Sapountzia*, V. Di Palmab, G. Zafeiropoulosc, H. Penchevd, M.Α. Verheijenb, 6 M. Creatoreb, F. Ublekovd, V. Sinigerskyd, W.M. Arnold Bikc, H.O.A. Fredrikssona, 7 M.N. Tsampasc, J.W. Niemantsverdrieta 8 9 a SynCat@DIFFER, Syngaschem BV, P.O. Box 6336, 5600 HH, Eindhoven, The Netherlands, 10 www.syngaschem.com 11 b Department of Applied Physics, Eindhoven University of Technology, 5600MB, Eindhoven, The Netherlands 12 c DIFFER, Dutch Institute For Fundamental Energy Research, De Zaale 20, 5612 AJ, Eindhoven, The Netherlands 13 d Institute of Polymers, Bulgarian Academy of Sciences, Acad. Georgi Bonchev 103A Str., BG 1113, Sofia, 14 Bulgaria 15 16 *Corresponding author: Foteini Sapountzi, email: [email protected], postal address: Syngaschem BV, P.O. 17 Box 6336, 5600 HH, Eindhoven, The Netherlands 18 19 20 Abstract: Alcohol electrolysis using polymeric membrane electrolytes is a promising route for 21 storing excess renewable energy in hydrogen, alternative to the thermodynamically limited water 22 electrolysis. By properly choosing the ionic agent (i.e. H+ or OH-) and the catalyst support, and 23 by tuning the catalyst structure, we developed membrane-electrode-assemblies which are 24 suitable for cost-effective and efficient alcohol electrolysis. Novel porous electrodes were 25 prepared by Atomic Layer Deposition (ALD) of Pt on a TiO2-Ti web of microfibers and were 26 interfaced to polymeric membranes with either H+ or OH- conductivity. Our results suggest that 27 alcohol electrolysis is more efficient using OH- conducting membranes under appropriate 28 operation conditions (high pH in anolyte solution). -

Understanding Anode Overpotential
UNDERSTANDING ANODE OVERPOTENTIAL Rebecca Jayne Thorne1, Camilla Sommerseth1, Ann Mari Svensson1, Espen Sandnes2, Lorentz Petter Lossius2, Hogne Linga2, and Arne Petter Ratvik1 1Dept. of Materials Science and Engineering, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway 2Hydro Aluminium, Årdal, Norway Keywords: Anode, carbon, electrochemistry, characterisation, overpotential. Abstract have not linked these coke properties to overpotential directly [2, 9-11]. It is likely that coke and anode physical properties will In aluminium electrolysis cells the anodic process is associated have an effect on overpotential, as the reactivity of carbons, both with a substantial overpotential. Industrial carbon anodes are electrochemical reactions and oxidation reactions, is generally produced from a blend of coke materials, but the effect of coke known to be related to microstructural parameters [12, 13]. In type on anodic overpotential has not been well studied. In this addition, the formation and release of CO2 bubbles also relies on work, lab-scale anodes were fabricated from single cokes and the surface microstructure [14]. electrochemical methods were subsequently used to determine the overpotential trend of the anode materials. Attempts were then In a previous study that served as preliminary work on a new made to explain these trends in terms of both the physical and project, the total overpotential of a range of carbon anodes chemical characteristics of the baked anodes themselves and their varying only in coke type was measured [15]. These anodes were raw materials. Routine coke and anode characterisation methods labeled 1 to 4, and a distinct overpotential trend was found with were used to measure properties such as impurity concentrations anode 4 having a lower overpotential than anode 1 by -2 and reactivity (to air and CO2), while non-routine characterisation approximately 200 mV at 1 A cm . -

Polymer Electrolyte Fuel Cell Lifetime Limitations: the Role of Electrocatalyst Degradation
V.H.1 Polymer Electrolyte Fuel Cell Lifetime Limitations: The Role of Electrocatalyst Degradation (A) Durability Deborah J. Myers (Primary Contact) and (B) Cost Xiaoping Wang (C) Performance Argonne National Laboratory 9700 S. Cass Avenue Lemont, IL 60439 Technical Targets Phone: (630) 252-4261 E-mail: [email protected] This project is conducting fundamental studies of platinum-based PEMFC cathode electrocatalyst DOE Technology Development Manager: degradation mechanisms. Insights gained from these Nancy Garland studies can be applied toward the definition of operating Phone: (202) 586-5673 conditions to extend PEMFC lifetimes and to the E-mail: [email protected] development of cathode electrocatalyst materials that meet the following DOE 2015 electrocatalyst durability Subcontractors: targets with voltage cycling: • Johnson Matthey Fuel Cells, Sonning Commons, United Kingdom • 5,000 hours (<80ºC) and 2,000 hours (>80°C), • United Technologies Research Center, • <40% loss of initial catalytic mass activity, and East Hartford, CT • Massachusetts Institute of Technology, Boston, MA • <30 mV loss at 0.8 A/cm² • University of Texas at Austin, Austin, TX • University of Wisconsin-Madison, Madison, WI Accomplishments Project Start Date: October 1, 2009 • Prepared Ketjen carbon-supported Pt nano-particle Project End Date: September 30, 2012 electrocatalysts (Pt/C) of varying particle size and incorporated these electrocatalysts into the cathodes of membrane-electrode assemblies (MEAs). • Quantified Pt/C catalyst oxygen reduction reaction Objectives (ORR) activity, electrochemically-active surface • Understand the role of cathode electrocatalyst area (ECA), and performance losses in a fuel cell degradation in the long-term loss of polymer as a function of initial Pt particle size and cell electrolyte membrane fuel cell (PEMFC) parameters (relative humidity, temperature, upper performance, potential limit, and cycling protocol). -
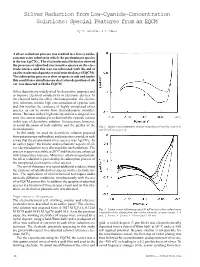
Silver Reduction from Low-Cyanide-Concentration Solutions: Special Features from an EQCM
Silver Reduction from Low-Cyanide-Concentration Solutions: Special Features from an EQCM By H. Sanchez & Y. Meas A silver reduction process was studied in a low-cyanide- concentration solution in which the predominant species - is the ion Ag(CN)2 . The electrochemical behavior showed the presence of adsorbed electroactive species on the elec- trode surface and this was corroborated with the aid of an electrochemical quartz crystal microbalance (EQCM). The adsorption process is slow at open circuit and under this condition a simultaneous electroless deposition of sil- ver was detected with the EQCM. Silver deposits are widely used for decorative purposes and to improve electrical conductivity in electronic devices. In the classical baths for silver electrodeposition, the electro- lytic solutions contain high concentrations of cyanide ions and this implies the existence of highly complexed silver species, as can be shown from thermodynamic consider- ations.1 Because of their high toxicity and for ecological rea- sons, the current tendency is to diminish the cyanide content in this type of electrolytic solution. It is necessary, however, to avoid alteration of bath stability and the quality of the Fig. 1—Single scan voltammetry of silver reduction at 10 mV/sec (curve 1) electrodeposits. and 20 mV/sec (curve 2). In this study, we used an electrolytic solution prepared from potassium pyrophosphate and potassium cyanide in such - a way that the predominant silver species was Ag(CN)2 . In an earlier paper,1 the kinetic and mechanistic aspects of sil- ver electroreduction were discussed for such solutions. The process is quasi-reversible at 20 °C and kinetics are enhanced with temperature increase. -

Direct Enzymatic Glucose/O2 Biofuel Cell Based on Poly-Thiophene Carboxylic Acid Alongside Gold Nanostructures Substrates Derive
www.nature.com/scientificreports OPEN Direct Enzymatic Glucose/O2 Biofuel Cell based on Poly- Thiophene Carboxylic Acid Received: 16 April 2018 Accepted: 18 September 2018 alongside Gold Nanostructures Published: xx xx xxxx Substrates Derived through Bipolar Electrochemistry Fereshte Gholami1, Aso Navaee1, Abdollah Salimi1,2, Rezgar Ahmadi2, Azam Korani1,3 & Rahman Hallaj1,2 Bipolar electrochemistry (BPE) has been lately explored as a simple, reliable and novel electrochemical technique for the adjustment of various conductive substrates. Herein, BPE is performed to derive both of cathode and anode electrodes for the development of mediatorless/membraneless biofuel cell (BFC). On one hand, a preferable substrate for immobilization of bilirubin oxidase enzyme is prepared based on the electropolymerization of thiophene-3-carboxcylic acid (TCA) on an Au microflm as a bipolar electrode. The resulted biocathode as novel bioelectrocatalyst ofers a high electrocatalytic activity toward direct oxygen reduction reaction (ORR) with onset potential and current density of 0.55 V (vs. Ag/AgCl) and 867 μA cm−2, respectively. On the other hand, another analogous Au bipolar electrode is electroplated through BPE to derive Au nanostructures (AuNSs). This modifed Au electrode is utilized as an anodic platform for immobilization of favin adenine dinucleotide-dependent glucose dehydrogenase (FAD-GDH) enzyme aimed at electrocatalytic glucose oxidation. The prepared bioanode displays a current density of 2.7 mA cm−2 with onset potential of −0.03 V. Finally, the proposed bioanode and biocacthode in an assembled membraneless glucose/O2 BFC ofers a power output of 146 μW cm−2 with open circuit voltage of 0.54 V. This novel BPE method provides disposable electrochemical platforms for design of novel sensors, biosensors or other devices.