Material Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Safety Data Sheet (English)
SAFETY DATA SHEET Diammonium Phosphate Prepared to U.S. OSHA, CMA, ANSI, Canadian WHMIS Standards, Australian WorkSafe, Japanese Industrial Standard JIS Z 7250:2000, Uruguay (Decree 307/2009 as amended by Decree 346/2011), SDS standards for Brazil (ABNT NRB 14725-4: 2014), and European Directives SECTION 1. PRODUCT IDENTIFICATION 1.1 TRADE NAME (AS LABELED): Diammonium Phosphate SYNONYMS: Ammonium Phosphate, Dibasic; Ammonium Phosphate, Secondary; DAP; Diammonium Phosphate Food grade; Diammonium Phosphate Tech Grade CAS#: 7783-28-0 EC NUMBER: 231-987-8 REACH REGISTRATION #: 01-2119490974-22-0057 1.2 PRODUCT USE: Nutrient in manufacture of yeast; ingredient in compound bread improvers. Flame retardant. pH regulator. Agriculture – Ingredient in specialty all-soluble dry fertilizers. Building Materials – Flame-proofing of wood. Paint – Ingredient in flame-proofing of specialty paper; prevention of afterglow in matches. Pulp and Paper – Flame-proofing of specialty paper; prevention of afterglow in materials. Textile – Flame-proofing of fabrics and cotton batting. Nutrient feed for biological treatment plants. 1.3 MANUFACTURER'S NAME: Innophos ADDRESS: 259 Prospect Plains Rd. Bldg A, Cranbury, NJ 08512 BUSINESS PHONE: 1-609-495-4295 WEB SITE INFORMATION: www.innophos.com RESPONSIBLE PARTY - EU Covance Clinical Development SA Parque Empresarial Las Tablas Edificio 1 Calle Federico Mompou 5-5ª planta 28050 Madrid, Spain Tel: +34 915 901 664 Email: [email protected] 1.4 EMERGENCY PHONE NUMBERS: 800-424-9300 (CHEMTREC U.S. and Canada – 24 Hrs) +1 703-527-3887 (CHEMTREC outside the USA and Canada – 24 Hrs) 615-386-7816 – Innophos Emergency Communication Team (ECT) 01-800-00214 00 (SETIQ in Mexico – 24 hrs) DATE OF PRIOR REVISION: November 16, 2017 DATE OF LATEST REVISION: February 09, 2020 SECTION 2. -

(DAP) Diammonium Phosphate Safety Data Sheet 200 Revision Date:04/30/2015 : Version: 1.0
(DAP) Diammonium phosphate Safety Data Sheet 200 Revision date:04/30/2015 : Version: 1.0 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Substance Substance name : (DAP) Diammonium phosphate Product code : DAP, DAPFR,DAPOS, DAPLG CAS No. : 7783-28-0 Formula : (NH4)2HPO4 Synonyms : Ammonium phosphate, dibasic / Diammonium hydrogenorthophosphate / Phosphoric acid, diammonium salt / Diammonium hydrogenphosphate / Ammonium phosphate dibasic / Diammonium hydrogen phosphate / Diammonium hydrogen orthophosphate / Phosphoric acid, ammonium salt (1:2) / DIAMMONIUM PHOSPHATE / DAP Product group : Commercial product Other means of identification : DAP, DAPLG 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/preparation : Agricultural chemical No additi onal infor mati on available 1.3. Details of the supplier of the safety data sheet PCS Sales (USA), Inc. 1101 Skokie Blvd. Suite 400 Northbrook, IL 60062 T 800-241-6908 / 847-849-4200 Suite 500 122 1st Avenue South Saskatoon, Saskatchewan Canada S7K7G3 T 800-667-0403 (Canada) / 800-667-3930 (USA) [email protected] - www.PotashCorp.com 1.4. Emergency telephone number Emergency number : 800-424-9300 CHEMTREC SECTION 2: Hazards identification 2.1. Classification of the substance or mixture GHS-US classification Skin Irrit. 2 H315 Eye Irrit. 2B H320 STOT SE 3 H335 Aquatic, Acute 2 H401 04/30/2015 EN (English) SDS Ref.: 200 1/10 (DAP) Diammonium phosphate Safety Data Sheet 200 Full text of H-phrases: see section 16 2.2. Label elements GHS-US labelling Hazard pictograms (GHS-US) : GHS07 Signal word (GHS-US) : Warning Hazard statements (GHS-US) : H315 - Causes skin irritation H320 - Causes eye irritation H335 - May cause respiratory irritation H401 - Toxic to aquatic life. -

Safety Data Sheet
Material name: Diammonium phosphate SDS BRAZIL SDS No. - Version #: 02 Issue date: 17-January-2013 Revision date: 24-October-2013 1 / 6 SAFETY DATA SHEET 1. Identification Name of the substance or Diammonium phosphate mixture (trade name) Synonyms Phosphoric acid, diammonium salt * Diammonium hydrogenorthophosphate * Diammonium hydrophosphate * DAP MSDS Number KFB_DAP_GHS_EN Major recommended uses for Fertilizer the substance or mixture Fertilizer. Specific restrictions for use of Not available. the substance or mixture Manufacturer/Importer/Distributor information Manufacturer Manufacturer/Supplier Koch Fertilizantes do Brasil Address Rua Joaquim Floriano 1.120 1 andar Cj. 12 Sao Paolo, SP BRASIL E-mail [email protected] Telephone +011 55 41 92263400 +1 316 828 7672 Emergency telephone For Chemical Emergency number Call CHEMTREC day or night USA/Canada - 1.800.424.9300 Outside USA/Canada 1.703.527.3887 (collect calls accepted) 2. Hazards identification Classification of the substance or mixture Physical hazards Not classified. Health hazards Not classified. Environmental hazards Not classified. Other hazards which do Not classified. not result in classification GHS labeling elements, including precautionary statements Hazard symbol(s) No symbol. Signal word No signal word. Hazard statement(s) Not available. Precautionary statement(s) Prevention Wear eye/face protection. Response Wash hands after handling. Storage Store away from incompatible materials. Disposal Dispose of waste and residues in accordance with local authority requirements. 3. Composition/information on ingredients Substance Common chemical name or technical name CAS number Concentration or concentration range Diammonium phosphate 7783-28-0 100 Material ID #: 902617 Material name: Diammonium phosphate SDS BRAZIL SDS No. - Version #: 02 Issue date: 17-January-2013 Revision date: 24-October-2013 2 / 6 Composition comments All concentrations are in percent by weight unless ingredient is a gas. -

Diammonium Phosphate (DAP)
Effective Date: July 1, 2003 Material Safety Data Sheet For Emergency Call: CHEMTREC -- (800) 424-9300 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Diammonium Phosphate (DAP) CAS Number: 7783-28-0 Product Uses: Agricultural industry: Fertilizer Industrial applications: Flame retardant agent. Corrosion inhibitors. Chemical Name: Ammonium phosphate, dibasic Chemical Family: Ammonium phosphates Synonyms and Common Trade Names: Ammonium phosphate; diammonium hydrogen phosphate; dibasic ammonium phosphate; secondary ammonium phosphate; DAP Company Identification: Manufacturer's Name: CF Industries, Inc. Address: One Salem Lake Drive, Long Grove, Illinois, 60047-8402 Telephone: (847) 438-9500 2. COMPOSITION/INFORMATION ON INGREDIENTS Typical Weight Component Name Percentage CAS Number Diammonium phosphate 60-85 7783-28-0 Aluminum ammonium phosphates 4-10 Not applicable Iron ammonium phosphates 3-5 Not applicable Ammonium sulfate 4-5 7783-20-2 Fluorides, as F 2-3 Not applicable Calcium ammonium phosphates 1-2 Not applicable Water 1-3 7732-18-5 Ammonium Nitrate <1 6484-52-2 Urea <1 57-13-6 Miscellaneous metal, ammonium and <1 each Not applicable other compounds Page 1 of 7 Effective Date: July 1, 2003 3. HAZARDS IDENTIFICATION Emergency Overview Caution! Eye and skin irritant. When heated to decomposition diammonium phosphate may emit toxic fumes of phosphorous oxides, nitrogen oxides and ammonia. Do not taste or swallow. Wash thoroughly after handling. Wear appropriate personal protection equipment. Slippery when wet. Brown to black granules that are odorless or give off a slight ammonia odor. Potential Health Effects: Eyes: Eye irritant. Contact may cause stinging, watering, redness and swelling. Skin: Skin irritant. Contact may cause redness, itching, burning and skin damage. -

SIAM 24, 17-20 April 2007 US/ICCA
SIAM 24, 17-20 April 2007 US/ICCA SIDS INITIAL ASSESSMENT PROFILE CAS Nos. 7722-76-1, 7783-28-0, 68333-79-9, 8011-76-5, 65996-95-4 Phosphate category: Monoammonium phosphate (MAP), Chemical Names Diammonium phosphate (DAP), Ammonium polyphosphate (APP), Single superphosphate (SSP), Triple superphosphate (TSP) MAP: NH4H2PO4 DAP: (NH4)2HPO4 Structural Formula APP: SSP: main components:Ca(H2PO4)2. H2O /CaSO4.H2O TSP: CaHPO4.2H2O SUMMARY CONCLUSIONS OF THE SIAR Category/Analogue Rationale The category consists of monoammonium phosphate (MAP; CAS No. 7722-76-1), diammonium phosphate (DAP; CAS No. 7783-28-0), ammonium polyphosphate (APP; CAS No. 68333-79-9), single superphosphate (SSP; CAS No. 8011-76-5), and triple superphosphate (TSP; CAS No. 65996-95-4). All members of the category are mainly or exclusively used as fertilizer and have one common functional group (phosphate) that equilibrates between several different ionic species - = = -3 [H3PO4, H2PO4 , HPO4 , HPO4 , or PO4 ] depending on the pH of the environment. Thus, chemical reactions for all compounds in this category are similar with the exception of the actual dissociation product, which forms calcium or ammonia along with common phosphate moieties. However, the presence of the ammonium ion will influence the observed toxicity and its data are used to conservatively represent the toxicity of the category members. Under typical environmental conditions, the phosphate would be present as monohydrogen = - - phosphate (HPO4 ) or dihydrogen phosphate (H2PO4 ) with the equilibrium favoring H2PO4 as the acidity of the environment increases. Under these conditions, the proportions of phosphoric acid -3 (H3PO4) and the phosphate anion (PO4 ) would be extremely low. -

MATERIAL SAFETY DATA SHEET DAP—Diammonium Phosphate Page 1 of 8
IMC MATERIAL SAFETY DATA SHEET DAP—Diammonium Phosphate Page 1 of 8 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: DAP—Diammonium Phosphate Chemical Name: Dibasic Ammonium Phosphate Chemical Family: Ammonium Phosphates—Inorganic Salt Synonyms/Brands: Ammonium Phosphate Dibasic DAP Fertilizer Grade Ammonium Phosphate 18-46-0 Chemical Formula: (NH4)2HPO4 Primary Use: Crop nutrient Responsible Party: IMC Phosphates 100 South Saunders Road, Suite 300 Lake Forest, IL 60045-2561 Non-Emergency 8:00 am – 4:00 pm Central Time, Mon - Fri: 800-323-5523 or 847-739-1200 Technical Contact: EMERGENCY OVERVIEW 24 Hour Emergency Telephone Number: For Chemical Emergencies: Spill, Leak, Fire or Accident Call CHEMTREC North America: (800) 424-9300 Others: (703)527-3887 (collect) Health Hazards: Eye and skin irritant. Avoid contact with eyes, skin and clothing. Wash thoroughly after handling. Physical Hazards: Slippery when wet. Physical Form: Solid. Appearance: Gray, tan, black granules. Odor: Slight ammonia odor. NFPA HAZARD CLASS HMIS HAZARD CLASS Health: 2 (Moderate) Health: 2 (Moderate) Flammability: 0 (Least) Flammability: 0 (Least) Instability: 0 (Least) Physical Hazard: 0 (Least) Special Hazard: None Status: Revised Issue Date: August 8, 2003 Revised Sections: 1, 2, 3, 4, 11, 12 MSDS # IGL 001 IMC MATERIAL SAFETY DATA SHEET DAP—Diammonium Phosphate Page 2 of 8 2. COMPOSITION/INFORMATION ON INGREDIENTS % Exposure Guideline Component Weight Limits Agency Type Diammonium Phosphate OSHA CAS No. 7783-28-0 85-87 NE All ACGIH (pure dibasic ammonium phosphate) Ammonium Nitrate OSHA <1.0 NE All CAS No. 6484-52-2 ACGIH Urea OSHA <1.0 NE All CAS No. -

Orca Corrosion Chart
Unsaturated Polyester Vinylster (Epoxy Acrylate Resins) CHEMICAL Conc Resins NO ISO BIS Novolac Bromine ENVIRONMENT % 511/512 301 585 570 545/555 A 1 Acetaldehyde 20 NR 40 40 40 2 Acetic Acid 10 80 100 100 100 3 Acetic Acid 15 60 100 100 100 4 Acetic Acid 25 60 100 100 100 5 Acetic Acid 50 - 80 80 80 6 Acetic Acid 75 NR 65 65 65 7 Acetic Acid, Glacial 100 NR NR 40 NR 8 Acetic Anhydride 100 NR NR 40 NR 9 Acetone 10 NR NR 80 80 10 Acetone 100 NR NR NR NR 11 Acetonitrile 20 - 40 40 40 12 Acetyl Acetone 20 - 40 50 40 13 Acrolein (Acrylaldehyde) 20 - 40 40 40 14 Acrylamide 50 NR 40 40 40 15 Acrylic Acid 25 NR 40 40 40 16 Acrylic Latex All - 80 80 80 17 Acrylonitrile Latex Dispersion 2 NR 25 25 25 Activated Carbon Beds, Water 18 - 80 100 80 Treatment Adipic Acid(1.5g solution in 19 23 - 80 80 80 water at 25℃, sol in hot water) 20 ALAMINE amines - 65 80 65 21 Alkyl(C8-10) Dimethyl Amine 100 - 80 100 80 22 Alkyl(C8-10) Chloride All - 80 100 95 23 Alkyl Benzene Sulfonic Acid 90 NR 50 50 50 Alkyl Tolyl Trimethyl 24 - - 40 50 40 Ammonium Chloride 25 Allyl Alcohol 100 NR NR 25 NR 26 Allyl Chloride All NR 25 25 25 27 Alpha Methylstyrene 100 NR 25 50 25 28 Alpha Oleum Sulfates 100 NR 50 50 50 29 Alum Sat'd 80 100 120 100 30 Aluminum Chloride Sat'd 80 100 120 100 31 Aluminum Chlorohydrate All - 100 100 100 32 Aluminum Chlorohydroxide 50 - 100 100 100 33 Aluminum Fluoride All - 25 25 25 34 Aluminum Hydroxide 100 80 80 95 80 35 Aluminum Nitrate All 80 100 100 100 36 Aluminum Potassium Sulfate Sat'd 80 100 120 100 37 Aluminum Sulfate Sat'd 80 100 120 100 -

Ammonium Phosphate, 40% W/V Safety Data Sheet According to Federal Register / Vol
Ammonium Phosphate, 40% w/v Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 08/13/2014 Revision date: 11/30/2016 Supersedes: 08/13/2014 Version: 1.1 SECTION 1: Identification 1.1. Identification Product form : Mixture Product name : Ammonium Phosphate, 40% w/v Product code : LC11295 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : For laboratory and manufacturing use only. Restrictions on use : Not for food, drug or household use 1.3. Details of the supplier of the safety data sheet LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Not classified 2.2. Label elements No labeling obligation. 2.3. Other hazards Other hazards not contributing to the : None under normal conditions. classification 2.4. Unknown acute toxicity (GHS US) Not applicable SECTION 3: Composition/Information on ingredients 3.1. Substance Not applicable 3.2. Mixture Name Product identifier % GHS -US classification Water (CAS No) 7732-18-5 60 Not classified DIAMMONIUM PHOSPHATE (CAS No) 7783-28-0 40 Not classified Full text of hazard classes and H-statements : see section 16 SECTION 4: First aid measures 4.1. -

An Access-Dictionary of Internationalist High Tech Latinate English
An Access-Dictionary of Internationalist High Tech Latinate English Excerpted from Word Power, Public Speaking Confidence, and Dictionary-Based Learning, Copyright © 2007 by Robert Oliphant, columnist, Education News Author of The Latin-Old English Glossary in British Museum MS 3376 (Mouton, 1966) and A Piano for Mrs. Cimino (Prentice Hall, 1980) INTRODUCTION Strictly speaking, this is simply a list of technical terms: 30,680 of them presented in an alphabetical sequence of 52 professional subject fields ranging from Aeronautics to Zoology. Practically considered, though, every item on the list can be quickly accessed in the Random House Webster’s Unabridged Dictionary (RHU), updated second edition of 2007, or in its CD – ROM WordGenius® version. So what’s here is actually an in-depth learning tool for mastering the basic vocabularies of what today can fairly be called American-Pronunciation Internationalist High Tech Latinate English. Dictionary authority. This list, by virtue of its dictionary link, has far more authority than a conventional professional-subject glossary, even the one offered online by the University of Maryland Medical Center. American dictionaries, after all, have always assigned their technical terms to professional experts in specific fields, identified those experts in print, and in effect held them responsible for the accuracy and comprehensiveness of each entry. Even more important, the entries themselves offer learners a complete sketch of each target word (headword). Memorization. For professionals, memorization is a basic career requirement. Any physician will tell you how much of it is called for in medical school and how hard it is, thanks to thousands of strange, exotic shapes like <myocardium> that have to be taken apart in the mind and reassembled like pieces of an unpronounceable jigsaw puzzle. -

THE EFFECTS of P FERTILIZER ADDITION on P TRANSFORMATIONS on HIGH-P FIXING and GRASSLAND SOILS by JOY PIERZYNSKI B.S., Michigan
THE EFFECTS OF P FERTILIZER ADDITION ON P TRANSFORMATIONS ON HIGH-P FIXING AND GRASSLAND SOILS by JOY PIERZYNSKI B.S., Michigan State University, 1982 M.S., Michigan State University, 1985 AN ABSTRACT OF A DISSERTATION submitted in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Department of Agronomy College of Agriculture KANSAS STATE UNIVERSITY Manhattan, Kansas 2016 Abstract Although phosphorus (P) is an essential nutrient for the growth of plants, it is one of the most limiting nutrients in terms of availability as a high proportion of applied P rapidly transforms into insoluble forms with low solubility in soils. To further understand the fate of P applied to soils, two separate but related studies using three high P-fixing soil types each were used for which the objectives were to investigate the mobility, availability, and reaction products from two granular and one liquid P fertilizer alone or plus a fertilizer enhancement product. Energy dispersive spectroscopy showed a substantial amount of P remained in the granule following a 5-week incubation. At the end of the 35-day incubation period there was evidence that the fluid fertilizer was superior over the granular sources in terms of enhanced diffusion and extractability of P for three calcareous soils with varying levels of CaCO3. Phosphorus x-ray absorption near-edge structure (XANES) spectroscopy results in conjunction with resin- extractable P indicated a strong negative correlation between Ca-P solids formed and P extractability, suggesting that degree of Ca-P formation limits P solubility. For the three acidic P- fixing soils the results were complex. -
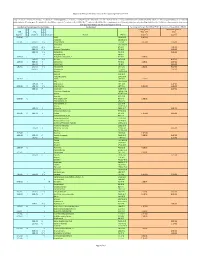
Regional Screening Level (RSL) Industrial Air Supporting Table June 2011
Regional Screening Level (RSL) Industrial Air Supporting Table June 2011 Key: I = IRIS; P = PPRTV; A = ATSDR; C = Cal EPA; X = PPRTV Appendix; H = HEAST; J = New Jersey; Y = New York; O = EPA Office of Water; E = Environmental Criteria and Assessment Office; S = see user guide Section 5; L = see user guide on lead; M = mutagen; V = volatile; F = See FAQ; c = cancer; * = where: n SL < 100X c SL; ** = where n SL < 10X c SL; n = noncancer; m = Concentration may exceed ceiling limit (See User Guide); s = Concentration may exceed Csat (See User Guide); SSL values are based on DAF=1 Toxicity and Chemical‐specific Information Contaminant Carcinogenic Target Risk (TR) = 1E‐06 Noncancer Hazard Index (HI) = 1 k k v Carcinogenic SL Noncarcinogenic SL IUR e RfCi e o TR=1.0E‐6 HI=1 (ug/m3)‐1 y (mg/m3) y c mutagen Analyte CAS No. (ug/m3) (ug/m3) 5.1E‐06 C ALAR 1596‐84‐5 2.4E+00 Acephate 30560‐19‐1 2.2E‐06 I 9.0E‐03 I V Acetaldehyde 75‐07‐0 5.6E+00 3.9E+01 Acetochlor 34256‐82‐1 3.1E+01 A V Acetone 67‐64‐1 1.4E+05 6.0E‐02 P V Acetone Cyanohydrin 75‐86‐5 2.6E+02 6.0E‐02 I V Acetonitrile 75‐05‐8 2.6E+02 V Acetophenone 98‐86‐2 1.3E‐03 C Acetylaminofluorene, 2‐ 53‐96‐3 9.4E‐03 2.0E‐05 I V Acrolein 107‐02‐8 8.8E‐02 1.0E‐04 I 6.0E‐03 I Acrylamide 79‐06‐1 1.2E‐01 2.6E+01 1.0E‐03 I Acrylic Acid 79‐10‐7 4.4E+00 6.8E‐05 I 2.0E‐03 I V Acrylonitrile 107‐13‐1 1.8E‐01 8.8E+00 6.0E‐03 P Adiponitrile 111‐69‐3 2.6E+01 Alachlor 15972‐60‐8 Aldicarb 116‐06‐3 Aldicarb Sulfone 1646‐88‐4 4.9E‐03 I Aldrin 309‐00‐2 2.5E‐03 Ally 74223‐64‐6 1.0E‐04 X Allyl Alcohol 107‐18‐6 4.4E‐01