8 Photosynthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chilling Out: the Evolution and Diversification of Psychrophilic Algae with a Focus on Chlamydomonadales
Polar Biol DOI 10.1007/s00300-016-2045-4 REVIEW Chilling out: the evolution and diversification of psychrophilic algae with a focus on Chlamydomonadales 1 1 1 Marina Cvetkovska • Norman P. A. Hu¨ner • David Roy Smith Received: 20 February 2016 / Revised: 20 July 2016 / Accepted: 10 October 2016 Ó Springer-Verlag Berlin Heidelberg 2016 Abstract The Earth is a cold place. Most of it exists at or Introduction below the freezing point of water. Although seemingly inhospitable, such extreme environments can harbour a Almost 80 % of the Earth’s biosphere is permanently variety of organisms, including psychrophiles, which can below 5 °C, including most of the oceans, the polar, and withstand intense cold and by definition cannot survive at alpine regions (Feller and Gerday 2003). These seemingly more moderate temperatures. Eukaryotic algae often inhospitable places are some of the least studied but most dominate and form the base of the food web in cold important ecosystems on the planet. They contain a huge environments. Consequently, they are ideal systems for diversity of prokaryotic and eukaryotic organisms, many of investigating the evolution, physiology, and biochemistry which are permanently adapted to the cold (psychrophiles) of photosynthesis under frigid conditions, which has (Margesin et al. 2007). The environmental conditions in implications for the origins of life, exobiology, and climate such habitats severely limit the spread of terrestrial plants, change. Here, we explore the evolution and diversification and therefore, primary production in perpetually cold of photosynthetic eukaryotes in permanently cold climates. environments is largely dependent on microbes. Eukaryotic We highlight the known diversity of psychrophilic algae algae and cyanobacteria are the dominant photosynthetic and the unique qualities that allow them to thrive in severe primary producers in cold habitats, thriving in a surprising ecosystems where life exists at the edge. -

157-21(5-20-00) Red Snow, Green Snow: It's Truly Spring When Those
May 20, 2000 BROWSE BROWSE TABLE OF BY YEAR 2000 ISSUES CONTENTS 2000 Vol. 157 No. 21 p. 328 Red Snow, Green Snow It’s truly spring when those last white drifts go technicolor By SUSAN MILIUS Microbiologist Brian Duval hates this part, so let’s just deal with the snickering up front. Yes, he studies yellow snow. He also studies red, green, and orange snow and would love to examine other colors if he were lucky enough to dis- cover them. His palette comes from springtime blooms of algae that live only in deep, persistent snowfields. And yes, even a professional sometimes gets fooled. “I was outside a penguin rookery in Antarctica, and I thought I was collecting this greenish-yellow algae,” recalls the microbiologist now at the Massachusetts Department of Environmental Protection in Worcester. “I was saying, ‘Yeah, yeah, this looks like the stuff.’” When he checked his trea- sures under a microscope, however, he diagnosed the obvious nonalgal origin. “It gets embarrassing,” he admits. Despite the yellow-snow raillery, Duval and his colleagues stay with their studies of snow algae because of the marvels of the chilly lifestyle. Somehow the 350 species of snow algae thrive in the near- freezing, nutrient-poor, acidic, sun-blasted slush of melting snowfields around the world. The algae support a food web in the snow—a world of tiny, wormy, crawly beings as odd as Spielberg-movie creatures. Duval Algal life cycles combine the drama of salmon runs and No, it’s not a murder scene. A the nightmare of icebound explorers. -

Study on Snowmelt and Algal Growth in the Antarctic Peninsula Using
RESEARCH COMMUNICATIONS Study on snowmelt and algal growth during February 2020 due to algal growth. Scientists from Ukraine have explained that due to red colour, snow in the Antarctic Peninsula using spatial reflects less sunlight and melts at a faster rate producing approach more bright algae7. Red snow was discovered by John Ross in 1818 at M. Geetha Priya1,*, N. Varshini2, G. Chandhana2, Crimson cliff in Baffin’s bay near the Arctic region. Later Robert Brown reported that the red colour was caused by G. Deeksha2, K. Supriya2 and D. Krishnaveni2 algae. At the beginning of the 20th century, Johan Nordal 1 School of Electronics Engineering, Vellore Institute of Technology, Fischer-Wille proved that the red colour of snow was due Chennai 600 127, India 2Centre for Incubation, Innovation, Research and Consultancy and to algae that he named Chlamydomonas nivalis (photo- 8,9 Department of ECE, Jyothy Institute of Technology, Thataguni, synthetic green algae) . Green algae grow in snowfields Bengaluru 560 082, India of the Alps and polar regions which consist of red carote- noid pigment that is responsible for the red colour pro- In recent times, global warming across the world is tecting it from UV radiation10,11. The present study is an one of the major factors that triggers surface snow immediate response to observations on the effect of rise melting. According to the reports, climate change in temperature on snow cover of Eagle Island and the across the globe has a major impact on the poles, change in snow reflectance due to red algal growth which which has resulted in rapid snowmelt and red- has been reported by polar researchers in February 2020. -

Morphological and Physicochemical Diversity of Snow Algae from Alaska Marta J
www.nature.com/scientificreports OPEN Morphological and physicochemical diversity of snow algae from Alaska Marta J. Fiołka1*, Nozomu Takeuchi2, Weronika Sofńska‑Chmiel3, Sylwia Mieszawska1 & Izabela Treska1 Snow algae are photosynthetic microbes growing in thawing snow. They usually show various morphological cell types. The aim of this study was to carry out microscopic and spectroscopic analysis of diferent forms of cells of snow algae collected on glaciers in Alaska. Four diferent shapes of algal cells were observed with the use of bright feld LM (Light Microscopy), DIC (Diferential Interference Contrast), EDF (Extended Depth Focus), fuorescence microscopy, and SEM (Scanning Electron Microscopy). The cells exhibited the strongest autofuorescence after the exposure to 365‑nm excitation light, and the intensity difered among the cell types. Zygotes (cysts) showed the most intense fuorescence. Acridine orange staining revealed the acid nature of the algal cells. The use of Congo red and Calcofuor white fuorochromes indicated diferences in the structure of polysaccharides in the cell wall in the individual types of algal cells. FTIR (Fourier‑Transform Infrared Spectroscopy) analyses showed the presence of polysaccharides not only in the algal cells but also in the fxative solution. The presence of polysaccharides in the extracellular algal fraction was confrmed by X‑ray dispersion spectroscopy (EDS), X‑ray photoelectron spectroscopy (XPS), and scanning electron microscopy imaging (SEM). The diferences observed in the structure of the cell wall of the diferent forms of red snow algae prompt further analysis of this structure. Te red or pink color of snow caused by blooms of snow algae is commonly observed during summer in alpine and coastal polar regions. -

Biological Albedo Reduction on Ice Sheets, Glaciers, and Snowfields
Biological albedo reduction on ice sheets, glaciers, and snowfields Scott Hotaling1, Stefanie Lutz2, Roman J. Dial3, Alexandre M. Anesio4, Liane G. Benning2,5, Andrew G. Fountain6, Joanna L. Kelley1, Jenine McCutcheon7, S. McKenzie Skiles8, Nozomu Takeuchi9, and Trinity L. Hamilton10 Affiliations: 1 School of Biological Sciences, Washington State University, Pullman, WA, USA 2 GFZ German Research Center for Geosciences, Telegrafenberg, 14473 Potsdam, Germany 3 Institute of Culture and Environment, Alaska Pacific University, Anchorage, AK, USA 4 Department of Environmental Science, Aarhus University, 4000 Roskilde, Denmark 5 Department of Earth Sciences, Free University of Berlin, 12249 Berlin, Germany 6 Departments of Geology and Geography, Portland State University, Portland, OR, USA 7 Department of Earth and Environmental Sciences, University of Waterloo, Waterloo, Canada 8 Department of Geography, University of Utah, Salt Lake City, UT, USA 9 Department of Earth Sciences, Graduate School of Science, Chiba University, Chiba, Japan 10 Department of Plant and Microbial Biology and The BioTechnology Institute, University of Minnesota, Saint Paul, MN, USA Correspondence: Scott Hotaling, School of Biological Sciences, Washington State University, Pullman, WA, 99164, USA; Email: [email protected]; Phone: (828) 507-9950; ORCID: 0000-0002-5965- 0986 Trinity Hamilton, Department of Plant and Microbial Biology and the BioTechnology Institute, University of Minnesota, Saint Paul, MN, USA; Email: [email protected]; Phone: (612) 625- 6372; ORCID: 0000-0002-2282-4655 Note: This article is a non-peer reviewed preprint submitted to EarthArXiv. It was submitted for peer review to Earth-Science Reviews in January 2021. 1 Abstract: 2 The global cryosphere, Earth’s frozen water, is in precipitous decline. -

Redacted for Privacy
AN ABSTRACT OF THE THESIS OF EMERY ALLEN SUTTON for the DOCTOR OF PHILOSOPHY (Name) (Degree) in OCEANOGRAPHY presented on ('7_//7 (Major) £1 (Dat'e) Title:THE PHYSIOLOGY AND LIFE HISTORIES OF SELECTED CRYOPHYTES OF. THE PACLFIC NORTHWEST Abstract approved: Redacted for Privacy The snow algae literature of the past 150 years is reviewed and a discussion of the possible biochemical reasons for cryophily are presented.The physiological responses to variation in light intensity, temperature, and nutrient media are presented for the following species: Chromulina chionophila Stein, Chiamydomonas nivalis (Bauer) Wille, Stichococcus bacillaris Nag., Raphidonema nivale Lagerh., and Chlamydomonas yellowstonesis Kol.Only C. chionophila is truly cryophilic.The others appear to be only cryobiontic.The morphology of C. chionophila and Chiamydomonas nivalis changes with variation in culture conditions.The life cycle of C. nivalis in culture is illustrated. Snow algae are found in the gut of the ice worm, Mesenchytraeus solifugus, and serve as food for rotifers in culture.The role of snow algae in the ecology of ice and snow fields is discussed. The Physiology and Life Histories of Selected Cryophytes of the Pacific Northwest by Emery Allen Sutton A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy June 1972 APPROVED: Redacted for Privacy Professor of Oceanography in charge of major Redacted for Privacy Chairmn of Departmenj of Ocean ã15hr- Redacted for Privacy Dean of Graduate School Date thesis is presented i,/97à Typed by Clover Redfern for Emery Allen Sutton ACKNOWLEDGMENT The number of individuals who deserve to be acknowledged for their aid in the completion of this work would comprise a list whose length would rival the length of the thesis.These individuals are already aware of my gratitude and to place their names and titles in this section appears, to me, to be unnecessary. -

Evaluation of Green Microalgae Biodiversity in the Alpine Ecosystem Adeline Stewart
Evaluation of green microalgae biodiversity in the alpine ecosystem Adeline Stewart To cite this version: Adeline Stewart. Evaluation of green microalgae biodiversity in the alpine ecosystem. Vegetal Biology. Université Grenoble Alpes [2020-..], 2021. English. NNT : 2021GRALV012. tel-03261557 HAL Id: tel-03261557 https://tel.archives-ouvertes.fr/tel-03261557 Submitted on 15 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE GRENOBLE ALPES Spécialité : Biologie Végétale Arrêté ministériel : 25 mai 2016 Présentée par Adeline STEWART Thèse dirigée par Eric MARECHAL, DR1, CNRS, et codirigée par Eric COISSAC, MCF, UGA et par Jean-Gabriel VALAY, PR, UGA préparée au sein du Laboratoire d’Ecologie Alpine et au Laboratoire de Physiologie Cellulaire et Végétale avec le soutien de l’unité mixte de service Lautaret dans l'École Doctorale de Chimie Sciences du Vivant Evaluation de la biodiversité des microalgues vertes dans l'écosystème alpin Thèse soutenue publiquement le 3 Mars 2021, devant le jury -

NEO-IAS-Daily-Newsletter-2Nd-March
NEO IAS Daily Newsletter TARGET PRELIMS 2020 (TPS 2020) Daily Prelims Current Affairs 2nd March, 2020 | Prelims Countdown: 89 Days Join our ECONOMY Prelims Telegram Channel: https://t.me/NEOIASECONOMYPRELIMS Join our Prelims Telegram Channel for Regular Updates: https://t.me/NEOIASPRELIMS Subscribe our YouTube channel for daily current affairs videos: www.youtube.com/neoias TABLE OF CONTENTS Main Topics Red Pandas Red Snow or Watermelon Snow Renewable Energy Management Centres (REMC) National Handicapped Finance Development Corporation (NHFDC) RaIDer-X List of migratory species For any further enqueries, kindly Current Affairs Capsules contact us on: 9446331522, 9188152205 Launch of multilingual Incredible India Note: We attempt to cover new issues website every day and drop the issues which are already covered. Therefore aspirants Previous Year Questions are advised to regularly follow NEO IAS daily current affairs material and video for a guaranteed success in Prelims 2020. NEO IAS daily current MAIN TOPICS affairs stands out from other daily current affairs by its exclusive UPSC RED PANDAS prelims focus on aspects that lie in and Why in news? beyond current affairs. Genetic study reveals the endangered red panda is actually two separate species. Red pandas: The red pandais a small arboreal mammal found in the forests of India, Nepal, Bhutan and the northern mountains of Myanmar and southern China. The Red Pandas are called “Living Fossils” as they are the only living member of the Ailuridae mammalian family. In India, it is found in Sikkim, western Arunachal Pradesh, Darjeeling district of West Bengal and parts of Meghalaya. It is also the state animal of Sikkim. -

The Taxonomy, Phylogeny, Biogeography and Ecology Of
FEMS Microbiology Ecology, 95, 2019, fiz064 doi: 10.1093/femsec/fiz064 Advance Access Publication Date: 10 May 2019 Research Article RESEARCH ARTICLE Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): the taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow Lenka Prochazkov´ a´ 1,*,†, Thomas Leya2,HedaKrˇ´ızkovˇ a´ 1 and Linda Nedbalova´ 1,3 1Charles University, Faculty of Science, Department of Ecology, Vinicnˇ a´ 7, 128 44 Prague 2, Czech Republic, 2Fraunhofer Institute for Cell Therapy and Immunology, Branch Bioanalytics and Bioprocesses IZI-BB, Extremophile Research & Biobank CCCryo, Am Muehlenberg 13, 14476 Potsdam-Golm, Germany and 3The Czech Academy of Sciences, Institute of Botany, Dukelska´ 135, Treboˇ n,ˇ 379 82, Czech Republic ∗Corresponding author: Charles University, Faculty of Science, Department of Ecology, Vinicnˇ a´ 7, 128 44, Prague 2, Czech Republic. Tel: +420 221951809; E-mail: [email protected] One sentence summary: Red and orange spherical cysts causing snow colouration across several continents were investigated with regards to their geographic distribution, ecology, ultrastructure and phylogeny; the cosmopolitan distribution of a new independent lineage Sanguina within the Chlamydomonadales was molecularly confirmed. †Lenka Prochazkov´ a,´ http://orcid.org/0000-0003-3995-6483 ABSTRACT Melting snowfields in polar and alpine regions often exhibit a red and orange colouration caused by microalgae. The diversity of these organisms is still poorly understood. We applied a polyphasic approach using three molecular markers and light and electron microscopy to investigate spherical cysts sampled from alpine mountains in Europe, North America and South America as well as from both polar regions. -
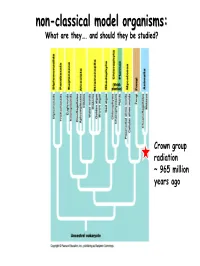
Non-Classical Model Organisms
nonnon--classicalclassical modelmodel organisms:organisms: What are they…. and should they be studied? Crown group radiation ~ 965 million years ago Classic eukaryote model organisms invertebrates & vertebrates invertebrates Arbacia punctulata, the purple-spined sea urchin, classical subject of embryological studies Aplysia, a sea slug, whose ink release response serves as a model in neurobiology and whose growth cones serve as a model of cytoskeletal rearrangements. Caenorhabditis elegans, a nematode, usually called C. elegans[2] - an excellent model for understanding the genetic control of development and physiology. C. elegans was the first multicellular organism whose genome was completely sequenced Ciona intestinalis, a sea squirt Drosophila, usually the species Drosophila melanogaster - a kind of fruit fly, famous as the subject of genetics experiments by Thomas Hunt Morgan and others. Easily raised in lab, rapid generations, mutations easily induced, many observable mutations. Recently, Drosophila has been used for neuropharmacological research[3]. (Molecular genetics, Population genetics, Developmental biology). Euprymna scolopes, the Hawaiian bobtail squid, model for animal-bacterial symbiosis, bioluminescent vibrios Hydra (genus), a Cnidarian, is the model organism to understand the evolution of bilaterian body plans Loligo pealei, a squid, subject of studies of nerve function because of its giant axon (nearly 1 mm diameter, roughly a thousand times larger than typical mammalian axons) Pristionchus pacificus, a roundworm used -

Ph.D Thesis Muhammad Rafiq Microbiology 2016.Pdf
Culture Dependent and Metagenomic study of Microbial Diversity of Glaciers in HKKH (Hindu Kush, Karakoram and Himalaya) mountain range By Muhammad Rafiq Department of Microbiology Quaid-i-Azam University Islamabad, Pakistan 2016 Culture Dependent and Metagenomic study of Microbial Diversity of Glaciers in HKKH (Hindu Kush, Karakoram and Himalaya) mountain range A thesis Submitted in the Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN MICROBIOLOGY By Muhammad Rafiq Department of Microbiology Quaid-i-Azam University Islamabad, Pakistan 2016 DECLARATION The material contained in this thesis is my original work and I have not presented any part of this thesis/work elsewhere for any other degree. Muhammad Rafiq DEDICATED TO My Ammi and Abbu CERTIFICATE This thesis, submitted by Mr. Muhammad Rafiq is accepted in its present form by the Department of Microbiology, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad as satisfying the thesis requirement for the degree of Doctor of Philosophy (PhD) in Microbiology. Internal Examiner: _______________________________ (Dr. Fariha Hasan) External Examiner: _____________________________ External Examiner: ______________________________ Chairperson: ____________________________________ Dated: CONTENTS S. No. Title Page No. 1. List of Abbreviations i 2. List of Tables ii 3. List of Figures iv 4. Acknowledgements vi 5. Summary viii 6. Chapter 1: Introduction 1 7. Chapter 2: Review of Literature 20 8. Chapter 3: Bacterial Diversity 89 9. Chapter 4: Fungal -

Iasbaba 60 Day Plan 2020 –Current Affairs
IASBABA 60 DAY PLAN 2020 –CURRENT AFFAIRS 60 DAYS PROGRAMME-2020 IASBABA IASBABA 60 DAY PLAN 2020 –CURRENT AFFAIRS Q.1) Which of the following statements with respect to ‘Ottan Thullal’. a) It is dance and poetic performance form of Kerala. b) It is a war dance performed in Karnataka c) It refers to colorful motifs done by hands in Tamil Nadu. d) It is a martial art of Andhra Pradesh which is similar to Silambam. Q.1) Solution (a) It is a dance and poetic performance form of Kerala, India. It was introduced in the eighteenth century by Kunchan Nambiar, one of the Prachina Kavithrayam (three famous Malayalam language poets). It is accompanied by a mridangam (a barrel shaped double headed drum) or an idakka (drum and cymbal). Q.2) Which of the following pairs is/are correctly matched? Places in News – State 1. Oussudu lake – Kerala 2. Sadikpur Sinauli – Rajasthan 3. Pari Adi mountain – Arunachal Pradesh Select the correct code: a) 1 Only b) 1 and 3 c) 2 and 3 d) 3 Only Q.2) Solution (d) Oussudu lake – Puducherry Sadikpur Sinauli – Uttar Pradesh Pari Adi mountain – Arunachal Pradesh Q.3) Which of the following countries do not open to the ‘Yellow Sea’? a) Japan b) South Korea c) North Korea d) China Q.3) Solution (a) 60 DAYS PROGRAMME-2020 IASBABA IASBABA 60 DAY PLAN 2020 –CURRENT AFFAIRS The Yellow Sea is a marginal sea of the Western Pacific Ocean located between mainland China and the Korean Peninsula, and can be considered the northwestern part of the East China Sea.